Iodine is prepared both in the laboratory and commercially by adding Cl2(g)Cl2(g) to an aqueous solution containing sodium iodide. 2NaI(aq)+Cl2(g)⟶I2(s)+2NaCl(aq) How many grams of sodium iodide, NaI,NaI, must be used to produce 34.5 g34.5 g of iodine, I2?
Iodine is prepared both in the laboratory and commercially by adding Cl2(g)Cl2(g) to an aqueous solution containing sodium iodide. 2NaI(aq)+Cl2(g)⟶I2(s)+2NaCl(aq) How many grams of sodium iodide, NaI,NaI, must be used to produce 34.5 g34.5 g of iodine, I2?
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter3: Calculations With Chemical Formulas And Equaitons
Section: Chapter Questions
Problem 3.93QP: Methanol, CH3OH, is prepared industrially from the gas-phase catalytic balanced reaction that has...
Related questions
Question
Iodine is prepared both in the laboratory and commercially by adding Cl2(g)Cl2(g) to an aqueous solution containing sodium iodide.
2NaI(aq)+Cl2(g)⟶I2(s)+2NaCl(aq)
How many grams of sodium iodide, NaI,NaI, must be used to produce 34.5 g34.5 g of iodine, I2?
Expert Solution
Step 1
The mass of the substance can be calculated if the molar mass and moles of that substance are known. The molarity of the solution is also related to moles. The moles can also be determined with the help of stoichiometry.
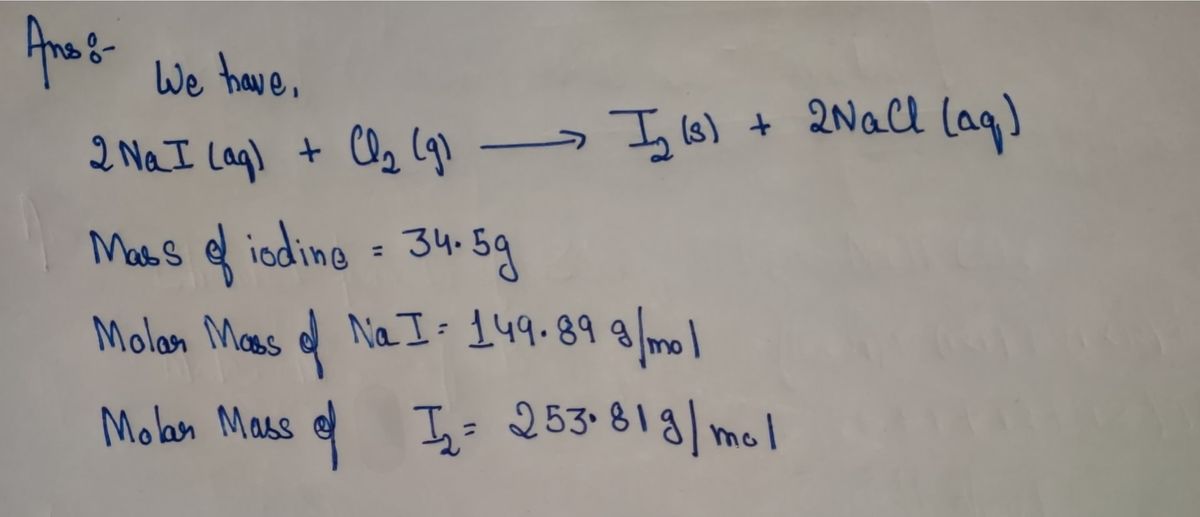
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning