Chapter14: Chromatography
Section: Chapter Questions
Problem 9P
Related questions
Question
A sample of oxygen gas has a volume of 3.18 L at 31°C. What volume will it occupy at 145°F if the pressure in number of moles are constant? 

Transcribed Image Text:onn. • ROKUTV (EID. m
CONFORMS TO UL
Model: 100012589
Power Input 100-240V-50/60, 4W
Use: DCSV
Intertek
3076343
500m
CAUTION
Unmr
E Essay #3-Korie Wallac X
Greece: Violence Agai X
G policed - Google Sear X
G ice - Google Search
NE Sexu:
ucation.com/ext/map/index.html?_con=con&external_browser=0&launchUrl=https%253A%252F%252Fnccu.blackboard.com
Saved
attempt left
Check my work
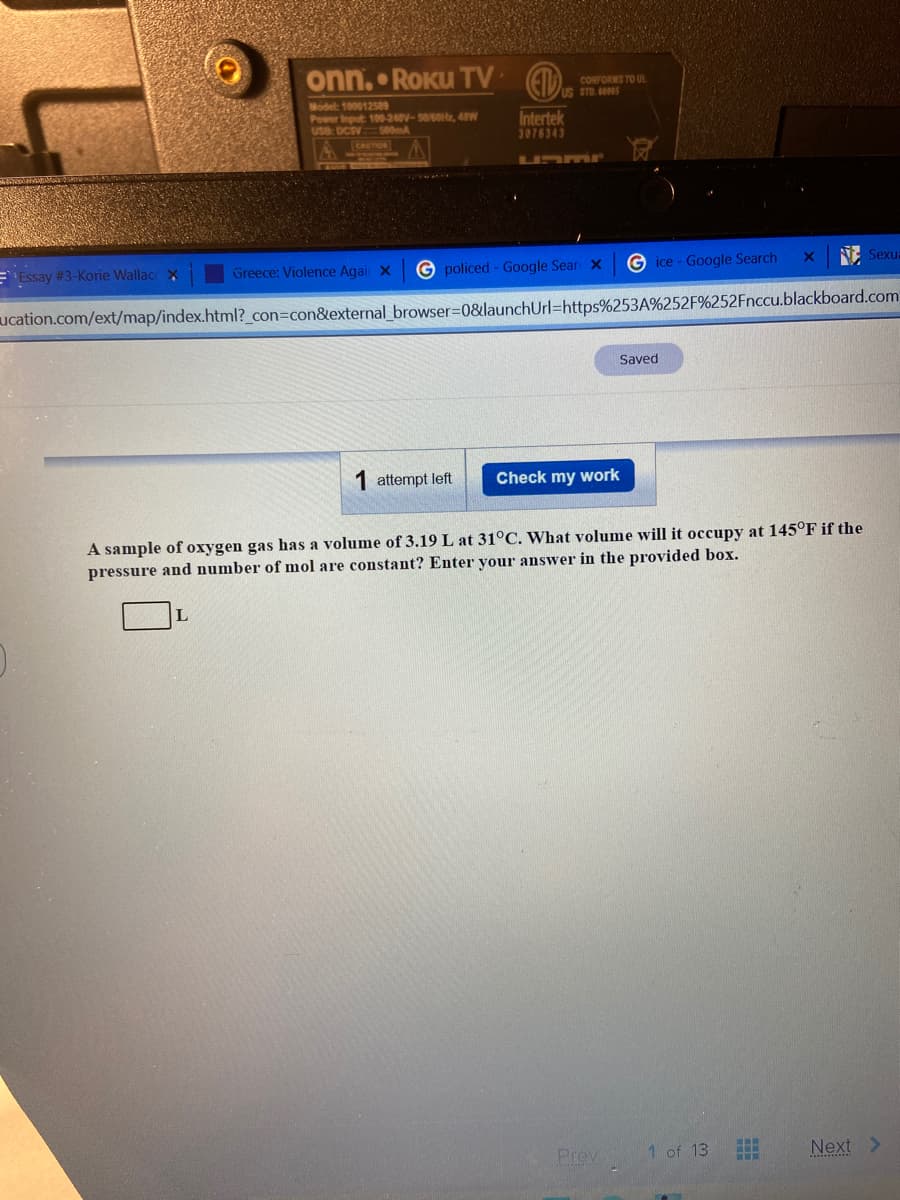
A sample of oxygen gas has a volume of 3.19 L at 31°C. What volume will it occupy at 145°F if the
pressure and number of mol are constant? Enter your answer in the provided box.
Prev
1 of 13
Next >
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



