A saturated solution of barium fluoride, BaF2, was prepared by dissolving solid BaF2 in water. The concentration of Ba2+ ion in the solution was found to be 7.52x103 M. Calculate Ksp for BaF2. Express your answer numerically. • View Available Hint(s) ? ΑΣφ Ksp %D II
A saturated solution of barium fluoride, BaF2, was prepared by dissolving solid BaF2 in water. The concentration of Ba2+ ion in the solution was found to be 7.52x103 M. Calculate Ksp for BaF2. Express your answer numerically. • View Available Hint(s) ? ΑΣφ Ksp %D II
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.60PAE
Related questions
Question
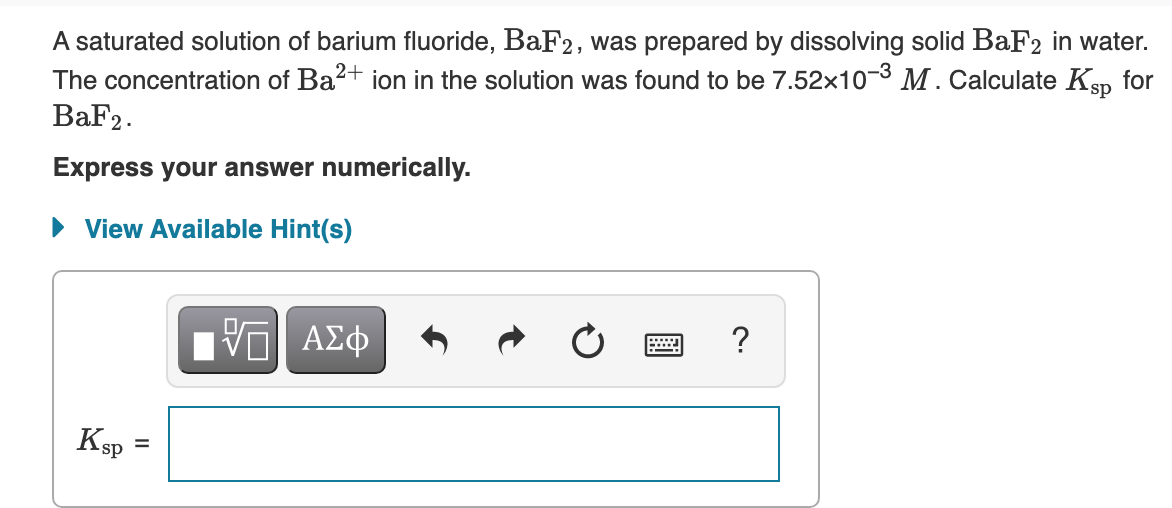
Transcribed Image Text:A saturated solution of barium fluoride, BaF2, was prepared by dissolving solid BaF2 in water.
The concentration of Ba2+ ion in the solution was found to be 7.52x103 M. Calculate Ksp for
BaF2.
Express your answer numerically.
• View Available Hint(s)
?
ΑΣφ
Ksp
%D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning