A scientist conducts an experiment to determine the rate of NO formation in the reaction: N2{g) + O2(g) → 2NO(g) If the initial concentration of N2 was 0.500 M and the concentration of N2 was 0.450 M after 0.100 s, what is the rate of NO formation? O 10.0 M/s O 1.00 M/s O 5.00 M/s O 0.500 M/s
A scientist conducts an experiment to determine the rate of NO formation in the reaction: N2{g) + O2(g) → 2NO(g) If the initial concentration of N2 was 0.500 M and the concentration of N2 was 0.450 M after 0.100 s, what is the rate of NO formation? O 10.0 M/s O 1.00 M/s O 5.00 M/s O 0.500 M/s
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter14: Chemical Kinetics: The Rates Of Chemical Reactions
Section: Chapter Questions
Problem 10PS: Nitrosyl bromide, NOBr, is formed from NO and Br2: 2 NO(g) + Br2(g) 2 NOBr(g) Experiments show that...
Related questions
Question
100%
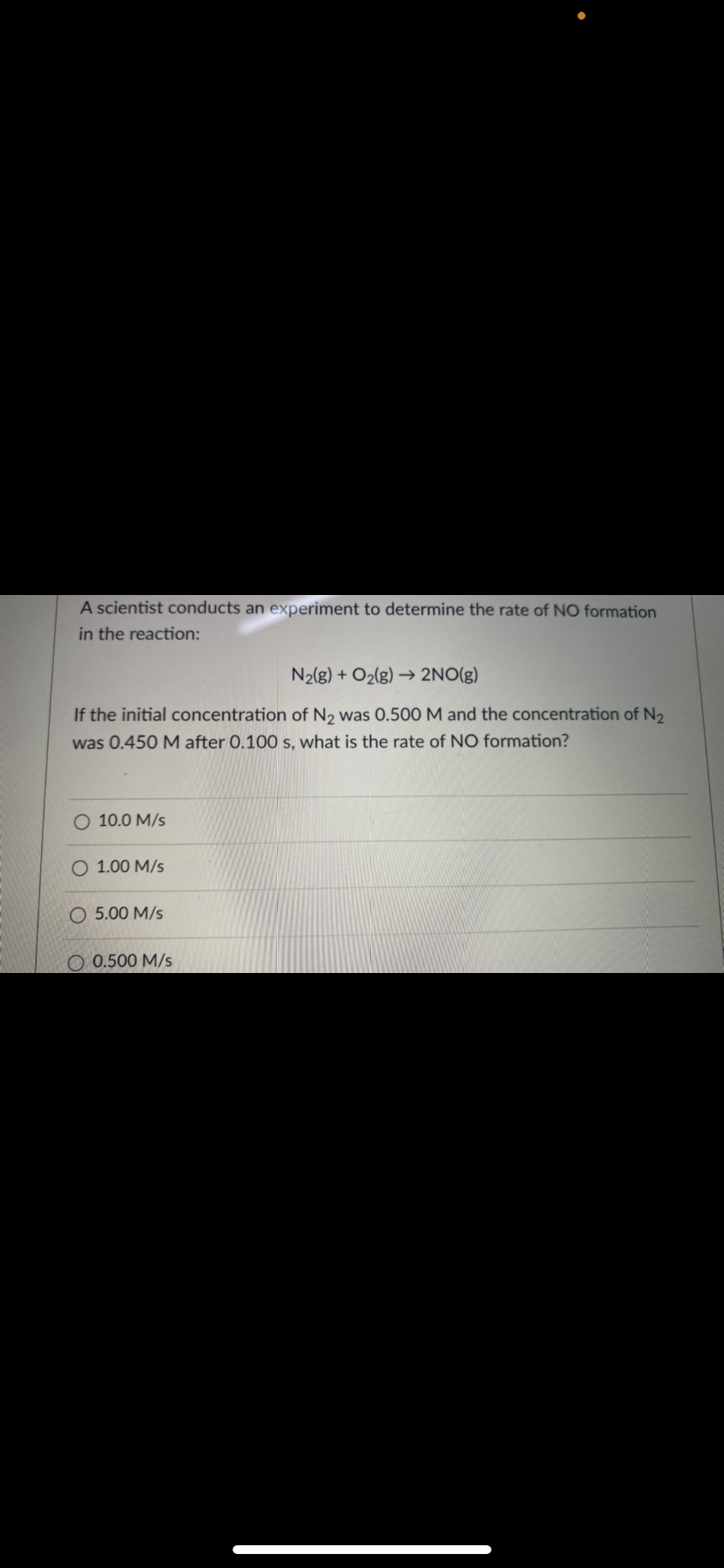
Transcribed Image Text:A scientist conducts an experiment to determine the rate of NO formation
in the reaction:
N2(g) + O2(g) 2NO(g)
If the initial concentration of N2 was 0.500M and the concentration of N2
was 0.450 M after 0.100 s, what is the rate of NO formation?
O 10.0 M/s
1.00 M/s
O 5.00 M/s
O 0.500 M/s
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning