A scientist is trying to discover information about an unknown metal in a compound. The formula for the compound is believed to be XBr3 where X is the unknown metal. The scientist determined that a 4.737 g sample of this compound contains 5.329×10−2 mol Br . Calculate the atomic mass of the unknown metal, X .
A scientist is trying to discover information about an unknown metal in a compound. The formula for the compound is believed to be XBr3 where X is the unknown metal. The scientist determined that a 4.737 g sample of this compound contains 5.329×10−2 mol Br . Calculate the atomic mass of the unknown metal, X .
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter2: Chemical Compounds
Section: Chapter Questions
Problem 76QRT: You have a pure sample of the antiseptic aminacrine, C13H10N2.
Calculate the mass in grams of...
Related questions
Question
A scientist is trying to discover information about an unknown metal in a compound. The formula for the compound is believed to be XBr3 where X is the unknown metal. The scientist determined that a 4.737 g sample of this compound contains 5.329×10−2 mol Br . Calculate the
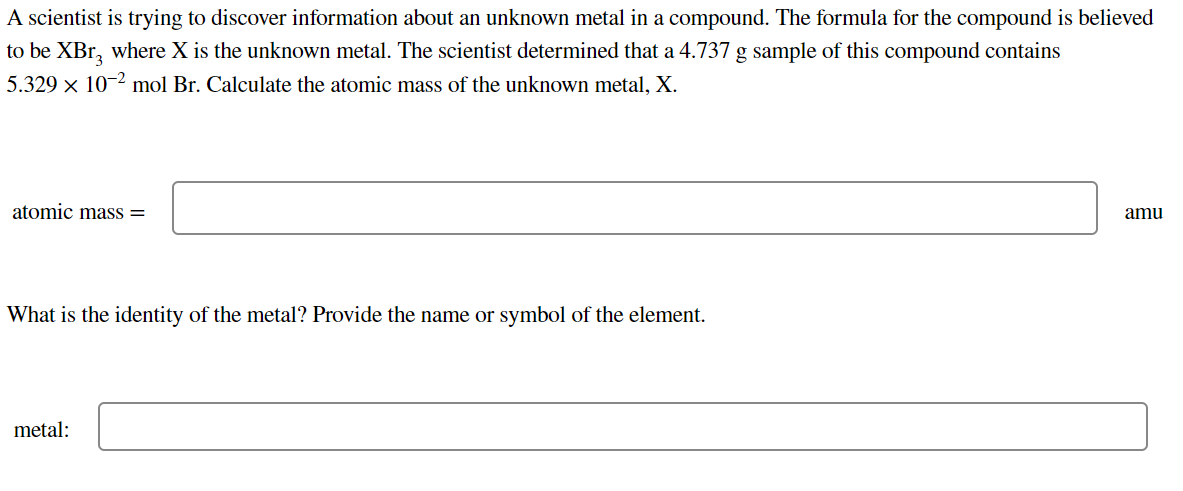
Transcribed Image Text:A scientist is trying to discover information about an unknown metal in a compound. The formula for the compound is believed
to be XBr, where X is the unknown metal. The scientist determined that a 4.737 g sample of this compound contains
5.329 x 10-2 mol Br. Calculate the atomic mass of the unknown metal, X.
atomic mass =
amu
What is the identity of the metal? Provide the name or symbol of the element.
metal:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
