(а) Show the electron counting for the metal center of each of the complexes below by using the ionic model. The answer should include the formal oxidation state of the metal, the number ofd electrons, ligand charges (if present) and the total electron count.
(а) Show the electron counting for the metal center of each of the complexes below by using the ionic model. The answer should include the formal oxidation state of the metal, the number ofd electrons, ligand charges (if present) and the total electron count.
Chapter26: Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 26.30QAP
Related questions
Question
Please do both
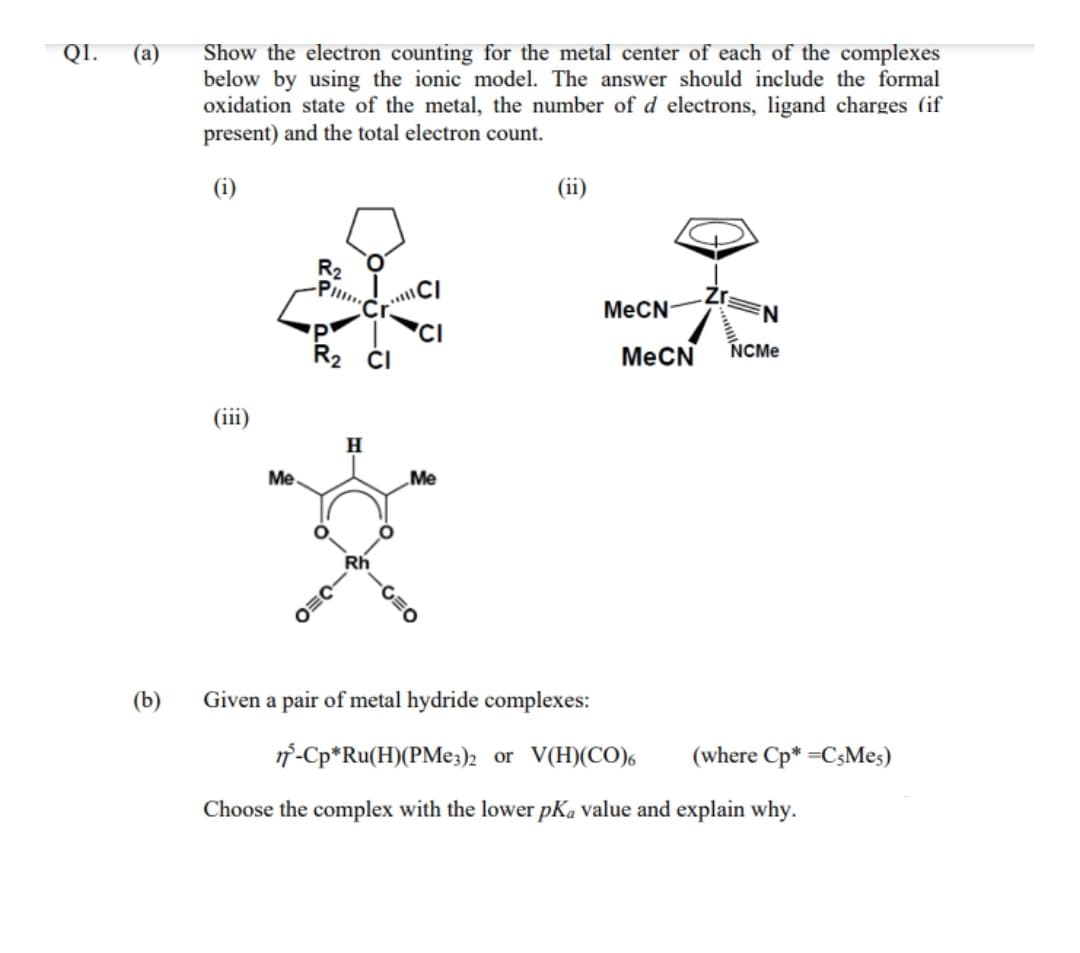
Transcribed Image Text:Show the electron counting for the metal center of each of the complexes
below by using the ionic model. The answer should include the formal
oxidation state of the metal, the number of d electrons, ligand charges (if
present) and the total electron count.
QI.
(а)
(i)
(ii)
R2
Pl
MECN-
MeCN
NCME
(iii)
Me
Me
Rh
(b)
Given a pair of metal hydride complexes:
1f-Cp*Ru(H)(PME3)2 or V(H)(CO),
(where Cp* =C3Mes)
Choose the complex with the lower pKa value and explain why.
-C=O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning