A slab of iron with temperature, Ta=48 deg C is used to heat a flat glass plate that has an initial temperature of Te=18 deg C. Assuming no heat is lost to the environment, and the masses are m=0.49 kg for the slab and mg= 310 g for the plate, what is the amount of heat transferred when the two have reached equal temperature? Assume c=0.11 kcal/kg-deg C for iron and cg=0.20 kcal/kg- deg C for glass.
A slab of iron with temperature, Ta=48 deg C is used to heat a flat glass plate that has an initial temperature of Te=18 deg C. Assuming no heat is lost to the environment, and the masses are m=0.49 kg for the slab and mg= 310 g for the plate, what is the amount of heat transferred when the two have reached equal temperature? Assume c=0.11 kcal/kg-deg C for iron and cg=0.20 kcal/kg- deg C for glass.
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter3: Transient Heat Conduction
Section: Chapter Questions
Problem 3.13P
Related questions
Question
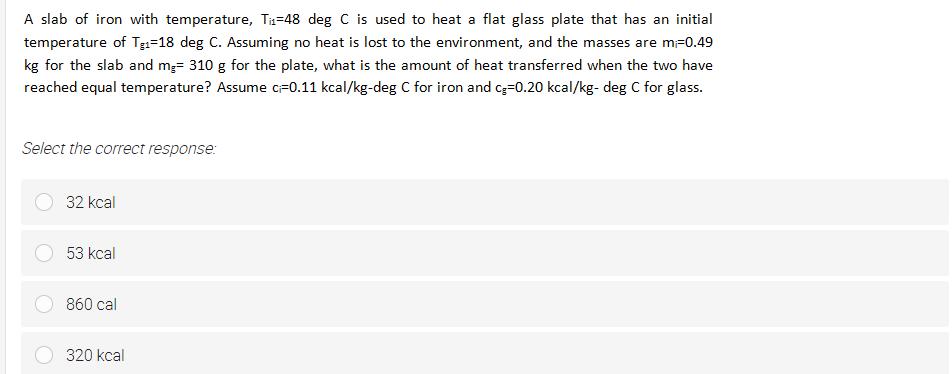
Transcribed Image Text:A slab of iron with temperature, Tz=48 deg C is used to heat a flat glass plate that has an initial
temperature of Tgi=18 deg C. Assuming no heat is lost to the environment, and the masses are m-0.49
kg for the slab and m;= 310 g for the plate, what is the amount of heat transferred when the two have
reached equal temperature? Assume cF0.11 kcal/kg-deg C for iron and cg=0.20 kcal/kg- deg C for glass.
Select the correct response:
32 kcal
53 kcal
860 cal
320 kcal
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning