A small object with mass 27.2 g is initially at 100.0°C. It is dropped into a coffee cup calorimeter containing 50.0 g of water at a temperature of 20.0°C. After stirring temperature of both object and water is 28.4 C. Assuming no heat losses, use the specific heat capacities below to identify the material the object is made of. H2O 4.184 J/g K 0.711 J/g K Al 0.900 J/g K Cu 0.387 J/g K Fe 0.450 J/g K Au 0.129 J/g K O Al O Cu O Au O Fe O o o O O
A small object with mass 27.2 g is initially at 100.0°C. It is dropped into a coffee cup calorimeter containing 50.0 g of water at a temperature of 20.0°C. After stirring temperature of both object and water is 28.4 C. Assuming no heat losses, use the specific heat capacities below to identify the material the object is made of. H2O 4.184 J/g K 0.711 J/g K Al 0.900 J/g K Cu 0.387 J/g K Fe 0.450 J/g K Au 0.129 J/g K O Al O Cu O Au O Fe O o o O O
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter7: Chemical Energy
Section: Chapter Questions
Problem 109AE: A sample of nickel is heated to 99.8C and placed in a coffee-cup calorimeter containing 150.0 g...
Related questions
Question
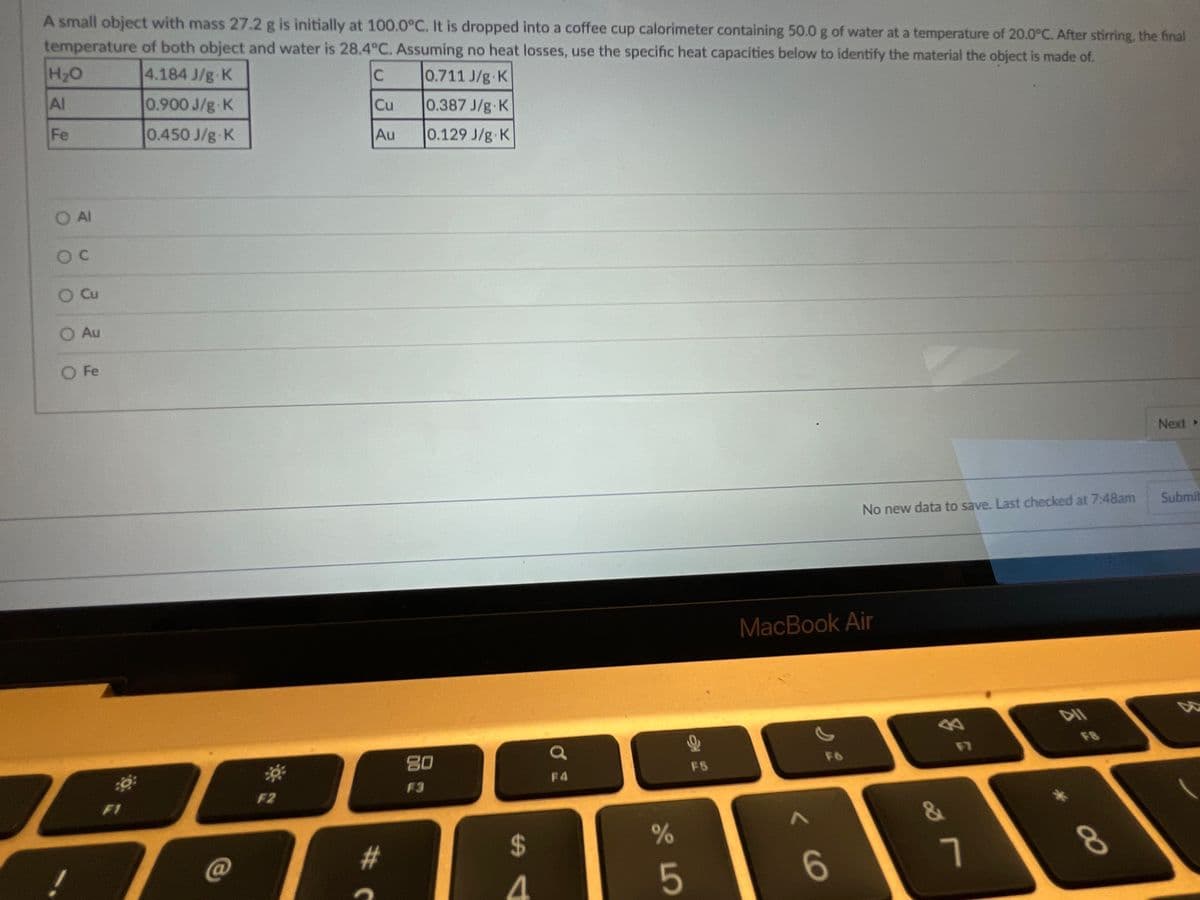
Transcribed Image Text:A small object with mass 27.2 g is initially at 100.0°C. It is dropped into a coffee cup calorimeter containing 50.0 g of water at a temperature of 20.0°C. After stirring, the final
temperature of both object and water is 28.4°C. Assuming no heat losses, use the specific heat capacities below to identify the material the object is made of.
H2O
4.184 J/g K
0.711 J/g K
Al
0.900 J/g-K
Cu
0.387 J/g K
Fe
0.450 J/g K
Au
0.129 J/g K
O Al
Cu
Au
O Fe
Next
No new data to save. Last checked at 7:48am
Submit
MacBook Air
公
F8
80
F7
F6
F5
F4
F3
F2
F1
24
4
C@
#
7
8.
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning