A solution containing a mixture of metal cations was treated as outlined. 1. Dilute HCI was added and a precipitate formed. The precipitate was filtered off. 2. H₂S was bubbled through the acidic solution. No precipitate formed. 3. The pH was raised to about 9 and H₂S was again bubbled through the solution. A precipitate formed and was filtered off. 4. Finally, sodium carbonate was added to the filtered solution and no precipitate formed. What can be said about the presence of each of these groups of cations in the original solution? Cation group Ag+, Pb²+, Hg2+ Bi³ +, Cd²+, Cu²+, Hg²+, Pb²+, Sb³ +, Sn²+, Sn4 + Al³+, Co²+, Cr³+, Fe²+, Fe³+, Ni²+, Mn²+, Zn²+ Ba²+, Ca²+, Mg²+, Sr² + Li+, Na+, K+, NH At least one of these ions was present. Description form insoluble chlorides form acid-insoluble sulfides form base-insoluble sulfides or hydroxides form insoluble carbonates completely soluble Answer Bank All of these ions were present. Present in the original solution? At least one of these ions was present. None of these ions were present. At least one of these ions was present. At least one of these ions was present. unknown None of these ions were present. unknown
A solution containing a mixture of metal cations was treated as outlined. 1. Dilute HCI was added and a precipitate formed. The precipitate was filtered off. 2. H₂S was bubbled through the acidic solution. No precipitate formed. 3. The pH was raised to about 9 and H₂S was again bubbled through the solution. A precipitate formed and was filtered off. 4. Finally, sodium carbonate was added to the filtered solution and no precipitate formed. What can be said about the presence of each of these groups of cations in the original solution? Cation group Ag+, Pb²+, Hg2+ Bi³ +, Cd²+, Cu²+, Hg²+, Pb²+, Sb³ +, Sn²+, Sn4 + Al³+, Co²+, Cr³+, Fe²+, Fe³+, Ni²+, Mn²+, Zn²+ Ba²+, Ca²+, Mg²+, Sr² + Li+, Na+, K+, NH At least one of these ions was present. Description form insoluble chlorides form acid-insoluble sulfides form base-insoluble sulfides or hydroxides form insoluble carbonates completely soluble Answer Bank All of these ions were present. Present in the original solution? At least one of these ions was present. None of these ions were present. At least one of these ions was present. At least one of these ions was present. unknown None of these ions were present. unknown
Chapter17: Complexation And Precipitation Reactions And Titrations
Section: Chapter Questions
Problem 17.35QAP
Related questions
Question
I have gotten this wrong 8 times! Please help. See screenshot attached. Thank you!
A solution containing a mixture of metal cations was treated as outlined.
- Dilute HClHCl was added and a precipitate formed. The precipitate was filtered off.
- H2SH2S was bubbled through the acidic solution. No precipitate formed.
- The pH was raised to about 9 and H2SH2S was again bubbled through the solution. A precipitate formed and was filtered off.
- Finally, sodium carbonate was added to the filtered solution and no precipitate formed.
What can be said about the presence of each of these groups of cations in the original solution?
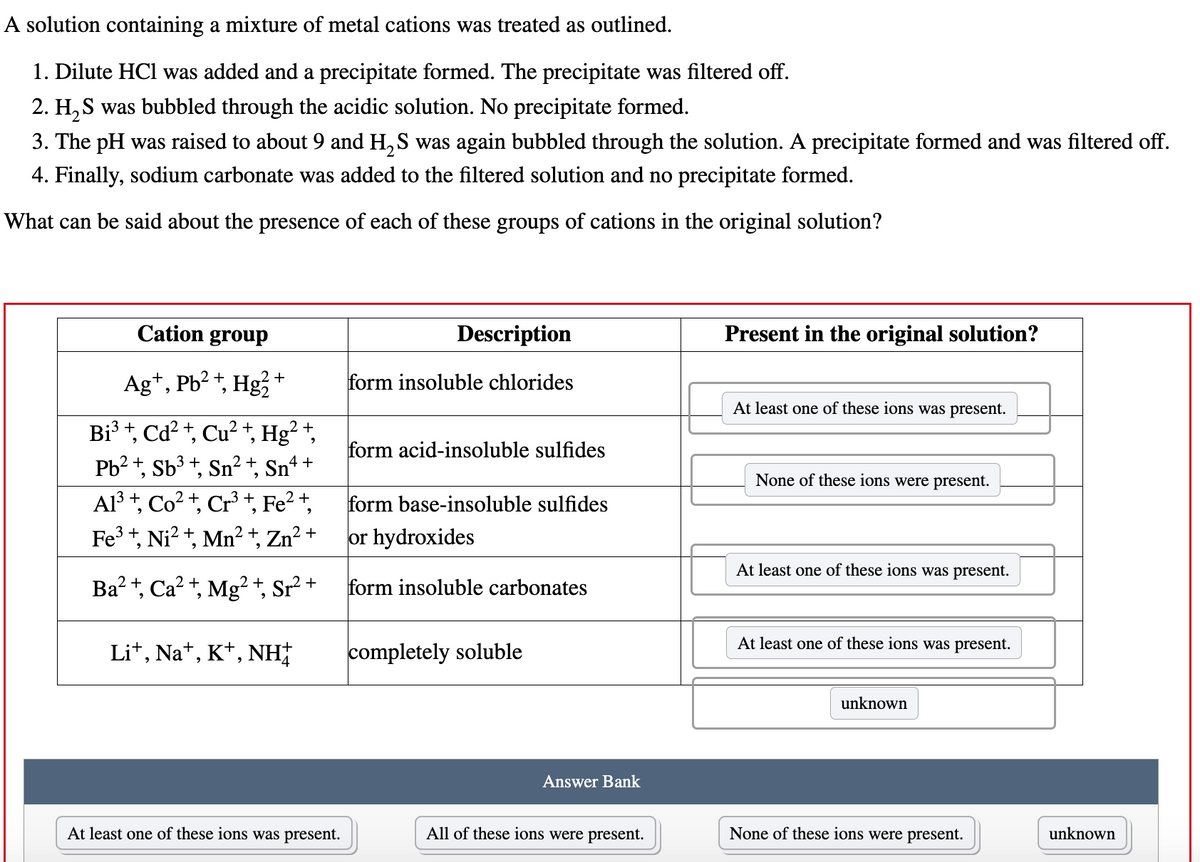
Transcribed Image Text:A solution containing a mixture of metal cations was treated as outlined.
1. Dilute HCl was added and a precipitate formed. The precipitate was filtered off.
2. H₂S was bubbled through the acidic solution. No precipitate formed.
3. The pH was raised to about 9 and H₂S was again bubbled through the solution. A precipitate formed and was filtered off.
4. Finally, sodium carbonate was added to the filtered solution and no precipitate formed.
What can be said about the presence of each of these groups of cations in the original solution?
Cation group
Ag+, Pb²+, Hg²+
Bi³ +, Cd²+, Cu²+,
Pb²+, Sb³ +, 2 + 4+
Sn² Snª
9
2 +
9
Hg²
Al³+, Co²+, Cr³+, Fe²+,
Fe³+, Ni²+, Mn²+, Zn²+
Ba²+, Ca²+, Mg²+, Sr² +
Li+, Na+, K+, NH
At least one of these ions was present.
Description
form insoluble chlorides
form acid-insoluble sulfides
form base-insoluble sulfides
or hydroxides
form insoluble carbonates
completely soluble
Answer Bank
All of these ions were present.
Present in the original solution?
At least one of these ions was present.
None of these ions were present.
At least one of these ions was present.
At least one of these ions was present.
unknown
None of these ions were present.
unknown
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax