A solution containing MgI2 is mixed with a solution of rubidium oxalate to form a solution that is 0.00035 M in magnesium ion and 0.000233 M in oxalate ion (C2O42–). What will happen once these solutions are mixed? Ksp (magnesium oxalate) = 2.3 × 10–9 Group of answer choices Nothing should happen because all species will be soluble. Nothing should happen because Q < Ksp for magnesium oxalate. The concentration of I– must be known in order to determine what will happen. A precipitate should form since Q > Ksp for magnesium oxalate. Nothing should happen because MgI2 and rubidium oxalate are soluble compounds.
A solution containing MgI2 is mixed with a solution of rubidium oxalate to form a solution that is 0.00035 M in magnesium ion and 0.000233 M in oxalate ion (C2O42–). What will happen once these solutions are mixed? Ksp (magnesium oxalate) = 2.3 × 10–9 Group of answer choices Nothing should happen because all species will be soluble. Nothing should happen because Q < Ksp for magnesium oxalate. The concentration of I– must be known in order to determine what will happen. A precipitate should form since Q > Ksp for magnesium oxalate. Nothing should happen because MgI2 and rubidium oxalate are soluble compounds.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 95AP: 95. Many metal ions form insoluble sulfide compounds when a solution of the metal ion is treated...
Related questions
Question
A solution containing MgI2 is mixed with a solution of rubidium oxalate to form a solution that is 0.00035 M in magnesium ion and 0.000233 M in oxalate ion (C2O42–). What will happen once these solutions are mixed?
Ksp (magnesium oxalate) = 2.3 × 10–9
Group of answer choices
Nothing should happen because all species will be soluble.
Nothing should happen because Q < Ksp for magnesium oxalate.
The concentration of I– must be known in order to determine what will happen.
A precipitate should form since Q > Ksp for magnesium oxalate.
Nothing should happen because MgI2 and rubidium oxalate are soluble compounds.
Expert Solution
Step 1
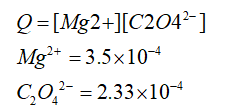
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning