Consider the following solubility data for acetanilide and aniline: Solubility per 100 mL water Acetinilide Cold Hot 0.6 5.0 Aniline 3.6 6.4 A mixture of 6.0 g acetinilide and 4.0 g aniline is to be separated using 100 mL of water as solvent. a. What mass of acetanilide remains in the mother liqour after hot filtration? b. What is the composition (in mass percent) of the crystals recovered after cold filtration? Show complete solution.
Consider the following solubility data for acetanilide and aniline: Solubility per 100 mL water Acetinilide Cold Hot 0.6 5.0 Aniline 3.6 6.4 A mixture of 6.0 g acetinilide and 4.0 g aniline is to be separated using 100 mL of water as solvent. a. What mass of acetanilide remains in the mother liqour after hot filtration? b. What is the composition (in mass percent) of the crystals recovered after cold filtration? Show complete solution.
Chapter10: Effect Of Electrolytes On Chemical Equilibria
Section: Chapter Questions
Problem 10.16QAP
Related questions
Question
Please don't just copy from other website :( I need the complete solution thank youuu
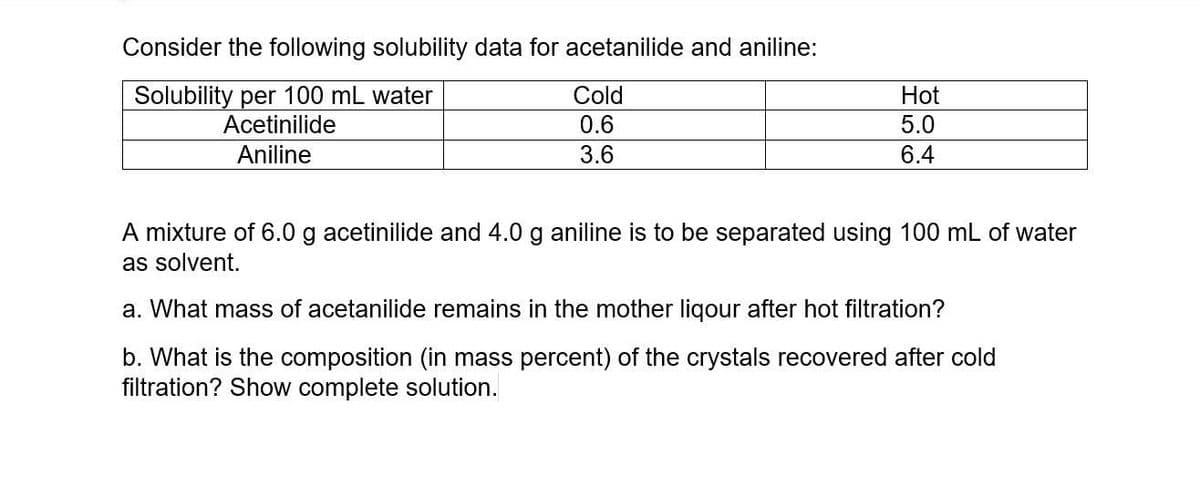
Transcribed Image Text:Consider the following solubility data for acetanilide and aniline:
Solubility per 100 mL water
Acetinilide
Cold
Hot
0.6
5.0
Aniline
3.6
6.4
A mixture of 6.0 g acetinilide and 4.0 g aniline is to be separated using 100 mL of water
as solvent.
a. What mass of acetanilide remains in the mother ligour after hot filtration?
b. What is the composition (in mass percent) of the crystals recovered after cold
filtration? Show complete solution.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT



EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning