A solution containing two different fluorescent compounds, Ben and Jerry, were analyzed for their individual concentrations in the mixture. Standards of pure Ben and pure Jerry were prepared at a concentration of 500.0 mM and were run in a UV-Vis Spectrophotometer to determine their absorption properties Absorbance Wavelength Compound Ben 500 mM Compound Jerry 500 mM 400 nm 0.137 0.136 450 nm 0.312 0.113 500 nm 0.154 0.078 550 nm 0.076 0.079 600 nm 0 227 0.148 650 nm 0230 0230 700 nm 0.151 0.357 750 nm 0.157 0246 800 nm 0.154 0.154 A standard curve of the standards was also prepared to help determine the concentration of each component in the solution. The solution produced an absorbance reading of 0.486 at the Amax of Ben, and 0.463 at the Amax of Jerry. Amax Jerry STD CURVE JERRY STD CURVE BEN Amax Ben Amax Ben Amаx Jerry CONC (mM) ABS ABS CONC (mM) ABS ABS 100 0.074 0.025 100 0.037 0.074 200 0.148 0.057 200 0.081 0.148 400 0.284 0.103 400 0.164 0 284 800 0.607 0218 S00 0.287 0.607 1600 1.457 0.499 1600 0.678 1.457 Using the given data, determine the following information: What is the Amax of Ben? O 750 nm O 400 nm O 450 nm O 650 nm İ..
A solution containing two different fluorescent compounds, Ben and Jerry, were analyzed for their individual concentrations in the mixture. Standards of pure Ben and pure Jerry were prepared at a concentration of 500.0 mM and were run in a UV-Vis Spectrophotometer to determine their absorption properties Absorbance Wavelength Compound Ben 500 mM Compound Jerry 500 mM 400 nm 0.137 0.136 450 nm 0.312 0.113 500 nm 0.154 0.078 550 nm 0.076 0.079 600 nm 0 227 0.148 650 nm 0230 0230 700 nm 0.151 0.357 750 nm 0.157 0246 800 nm 0.154 0.154 A standard curve of the standards was also prepared to help determine the concentration of each component in the solution. The solution produced an absorbance reading of 0.486 at the Amax of Ben, and 0.463 at the Amax of Jerry. Amax Jerry STD CURVE JERRY STD CURVE BEN Amax Ben Amax Ben Amаx Jerry CONC (mM) ABS ABS CONC (mM) ABS ABS 100 0.074 0.025 100 0.037 0.074 200 0.148 0.057 200 0.081 0.148 400 0.284 0.103 400 0.164 0 284 800 0.607 0218 S00 0.287 0.607 1600 1.457 0.499 1600 0.678 1.457 Using the given data, determine the following information: What is the Amax of Ben? O 750 nm O 400 nm O 450 nm O 650 nm İ..
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 4P
Related questions
Question
Transcribed Image Text:Question 22
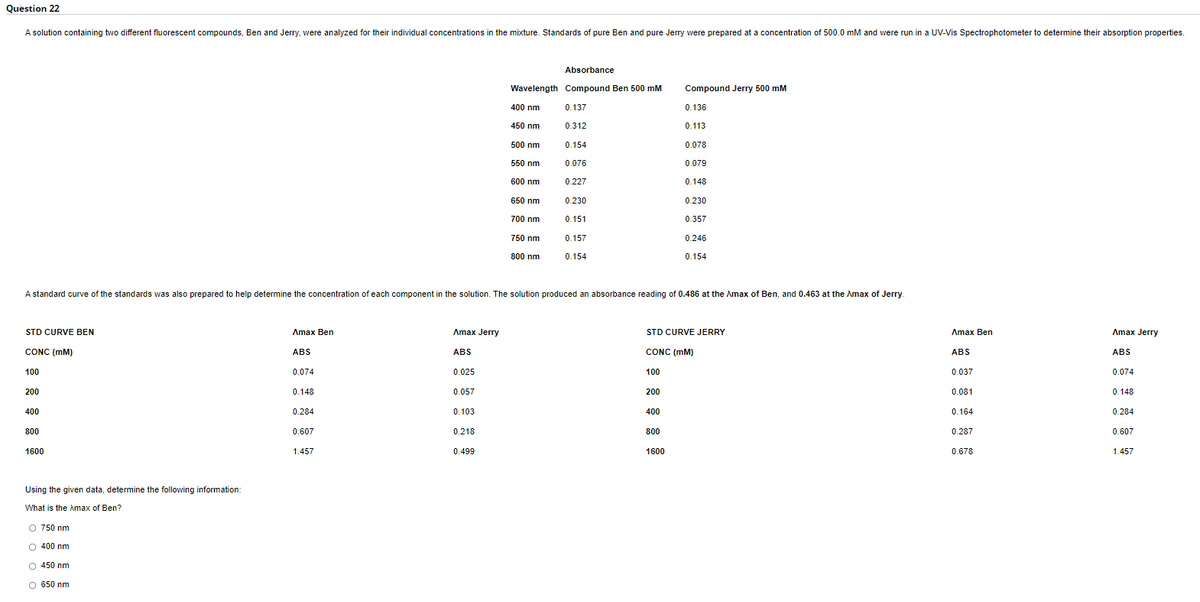
A solution containing two different fluorescent compounds, Ben and Jerry, were analyzed for their individual concentrations in the mixture. Standards of pure Ben and pure Jerry were prepared at a concentration of 500.0 mM and were run in a UV-Vis Spectrophotometer to determine their absorption properties.
Absorbance
Wavelength Compound Ben 500 mM
Compound Jerry 500 mM
400 nm
0.137
0.136
450 nm
0.312
0.113
500 nm
0.154
0.078
550 nm
0.076
0.079
600 nm
0.227
0.148
650 nm
0.230
0.230
700 nm
0.151
0.357
750 nm
0.157
0.246
800 nm
0.154
0.154
A standard curve of the standards was also prepared to help determine the concentration of each component in the solution. The solution produced an absorbance reading of 0.486 at the Amax of Ben, and 0.463 at the Amax of Jerry.
STD CURVE BEN
Amax Jerry
Amax Jerry
Лтax Ben
STD CURVE JERRY
Aтax Ben
CONC (mM)
ABS
ABS
CONC (mM)
ABS
ABS
100
0.074
0.025
100
0.037
0.074
200
0.148
0.057
200
0.081
0.148
400
0.284
0.103
400
0.164
0.284
800
0.607
0.218
800
0.287
0.607
1600
1.457
0.499
1600
0.678
1.457
Using the given data, determine the following information:
What is the Amax of Ben?
O 750 nm
O 400 nm
O 450 nm
O 650 nm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning