A solution contains 8.61×103 M silver acetate and 9.23×10-³ M calcium nitrate. Solid sodium chromate is added slowly to this mixture. A. What is the formula of the substance that precipitates first? formula = B. What is the concentration of chromate ion when this precipitation first begins? [Cro,²) =| M A solution contains 6.79×103 M magnesium nitrate and 1.30×10² M barium acetate. Solid potassium fluoride is added slowly to this mixture. A. What is the formula of the substance that precipitates first? formula = B. What is the concentration of fluoride ion when this precipitation first begins? [F°] = M
A solution contains 8.61×103 M silver acetate and 9.23×10-³ M calcium nitrate. Solid sodium chromate is added slowly to this mixture. A. What is the formula of the substance that precipitates first? formula = B. What is the concentration of chromate ion when this precipitation first begins? [Cro,²) =| M A solution contains 6.79×103 M magnesium nitrate and 1.30×10² M barium acetate. Solid potassium fluoride is added slowly to this mixture. A. What is the formula of the substance that precipitates first? formula = B. What is the concentration of fluoride ion when this precipitation first begins? [F°] = M
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter17: Principles Of Chemical Reactivity: Other Aspects Of Aqueous Equilibria
Section: Chapter Questions
Problem 96GQ: A solution contains 0.10 M iodide ion, I, and 0.10 M carbonate ion, CO32. (a) If solid Pb(NO3)2 is...
Related questions
Question
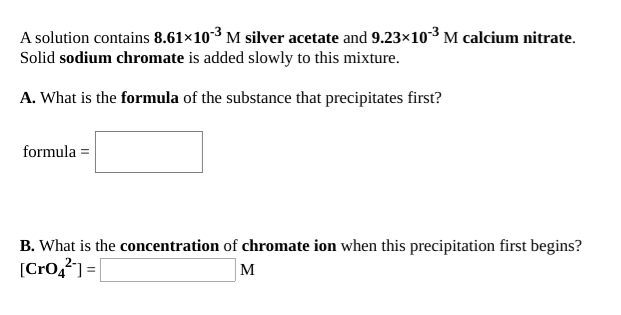
Transcribed Image Text:A solution contains 8.61×103 M silver acetate and 9.23×10-³ M calcium nitrate.
Solid sodium chromate is added slowly to this mixture.
A. What is the formula of the substance that precipitates first?
formula =
B. What is the concentration of chromate ion when this precipitation first begins?
[Cro,²) =|
M
![A solution contains 6.79×103 M magnesium nitrate and 1.30×10² M barium acetate.
Solid potassium fluoride is added slowly to this mixture.
A. What is the formula of the substance that precipitates first?
formula =
B. What is the concentration of fluoride ion when this precipitation first begins?
[F°] =
M](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F4a466984-9f97-4857-b5af-042e2bf7c34f%2F2ef230ab-2a86-43ad-927e-405f1c2c6916%2Fq905byc.png&w=3840&q=75)
Transcribed Image Text:A solution contains 6.79×103 M magnesium nitrate and 1.30×10² M barium acetate.
Solid potassium fluoride is added slowly to this mixture.
A. What is the formula of the substance that precipitates first?
formula =
B. What is the concentration of fluoride ion when this precipitation first begins?
[F°] =
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning