A solution is made by dissolving 15.1 g of ammonium fluoride, NH4F, in enough water to make exactly 100 mL of solution. Calculate the concentration (molarity) of NH4F in mol/L (M). MΝH4F Show Approach Show Tutor Steps Submit
A solution is made by dissolving 15.1 g of ammonium fluoride, NH4F, in enough water to make exactly 100 mL of solution. Calculate the concentration (molarity) of NH4F in mol/L (M). MΝH4F Show Approach Show Tutor Steps Submit
Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.106E
Related questions
Question
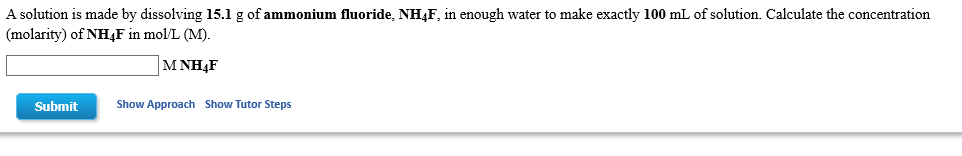
Transcribed Image Text:A solution is made by dissolving 15.1 g of ammonium fluoride, NH4F, in enough water to make exactly 100 mL of solution. Calculate the concentration
(molarity) of NH4F in mol/L (M).
MΝH4F
Show Approach
Show Tutor Steps
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning