Ô https://openvellum.ecollege.com/course.html?courseld=16516363&OpenVellumHMAC=656b563889ad890e249f777ac081d4f. o ... I Review | Constants | Periodic Table You may want to reference (Pages 298 - 306) Section 9.4 while completing this problem Part A Calculate the molarity of 0.550 mol of glucose in 0.250 L of a glucose solution. Calculate the molarity of the following solutions. Express your answer with the appropriate units. HẢ molarity = Value Units Submit Previous Answers Request Answer X Incorrect; Try Again; 2 attempts remaining Part B Calculate the molarity of 70.0 g of HCl in 1.50 L of an HCl solution. Express your answer with the appropriate units. ? P Pearson Copyright © 2021 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy | Permissions | Contact Us | 12:00 AM search (? 4/30/2021
Ô https://openvellum.ecollege.com/course.html?courseld=16516363&OpenVellumHMAC=656b563889ad890e249f777ac081d4f. o ... I Review | Constants | Periodic Table You may want to reference (Pages 298 - 306) Section 9.4 while completing this problem Part A Calculate the molarity of 0.550 mol of glucose in 0.250 L of a glucose solution. Calculate the molarity of the following solutions. Express your answer with the appropriate units. HẢ molarity = Value Units Submit Previous Answers Request Answer X Incorrect; Try Again; 2 attempts remaining Part B Calculate the molarity of 70.0 g of HCl in 1.50 L of an HCl solution. Express your answer with the appropriate units. ? P Pearson Copyright © 2021 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy | Permissions | Contact Us | 12:00 AM search (? 4/30/2021
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter6: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 123CP: The units of parts per million (ppm) and parts per billion (ppb) are commonly used by environmental...
Related questions
Question
100%
Transcribed Image Text:ô https://openvellum.ecollege.com/course.html?courseld=16516363&OpenVellumHMAC=656b563889ad890e249f777ac081d4f.
...
II Review | Constants | Periodic Table
Scores
You may want to reference
(Pages 298 - 306) Section 9.4 while completing this
problem.
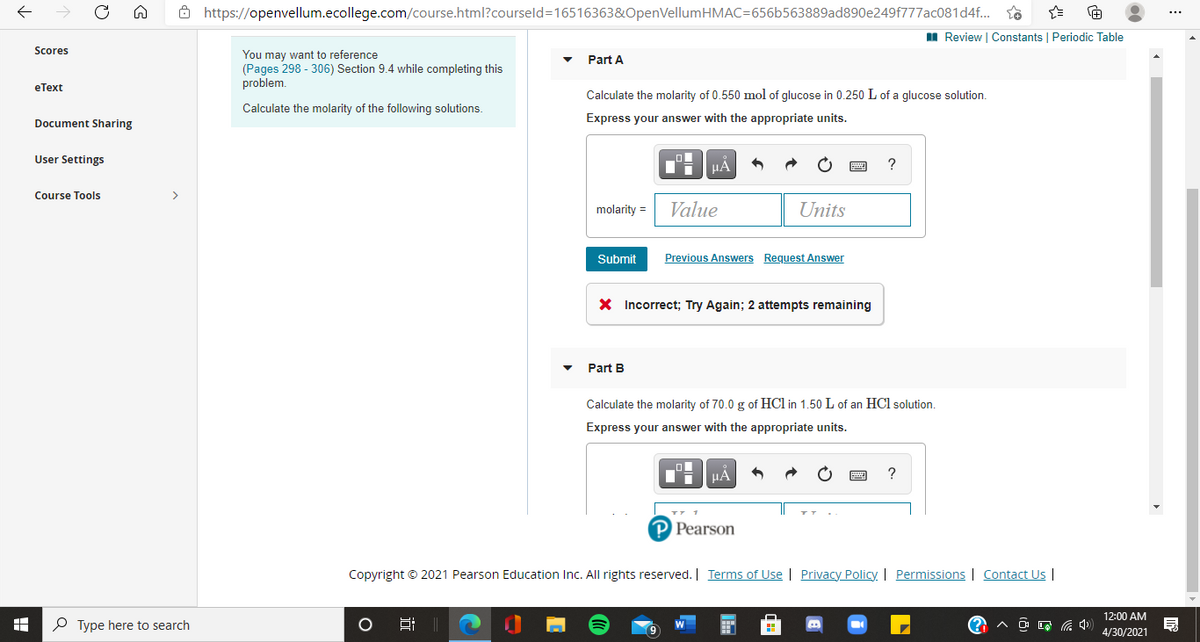
Part A
eТеxt
Calculate the molarity of 0.550 mol of glucose in 0.250 L of a glucose solution.
Calculate the molarity of the following solutions.
Document Sharing
Express your answer with the appropriate units.
User Settings
HA
?
Course Tools
>
molarity =
Value
Units
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 2 attempts remaining
Part B
Calculate the molarity of 70.0 g of HCl in 1.50 L of an HCl solution.
Express your answer with the appropriate units.
HA
P Pearson
Copyright © 2021 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy. | Permissions | Contact Us |
12:00 AM
P Type here to search
4/30/2021
Transcribed Image Text:I Review | Constants | Periodic Table
Scores
You may want to reference
(Pages 298 - 306) Section 9.4 while completing this
problem.
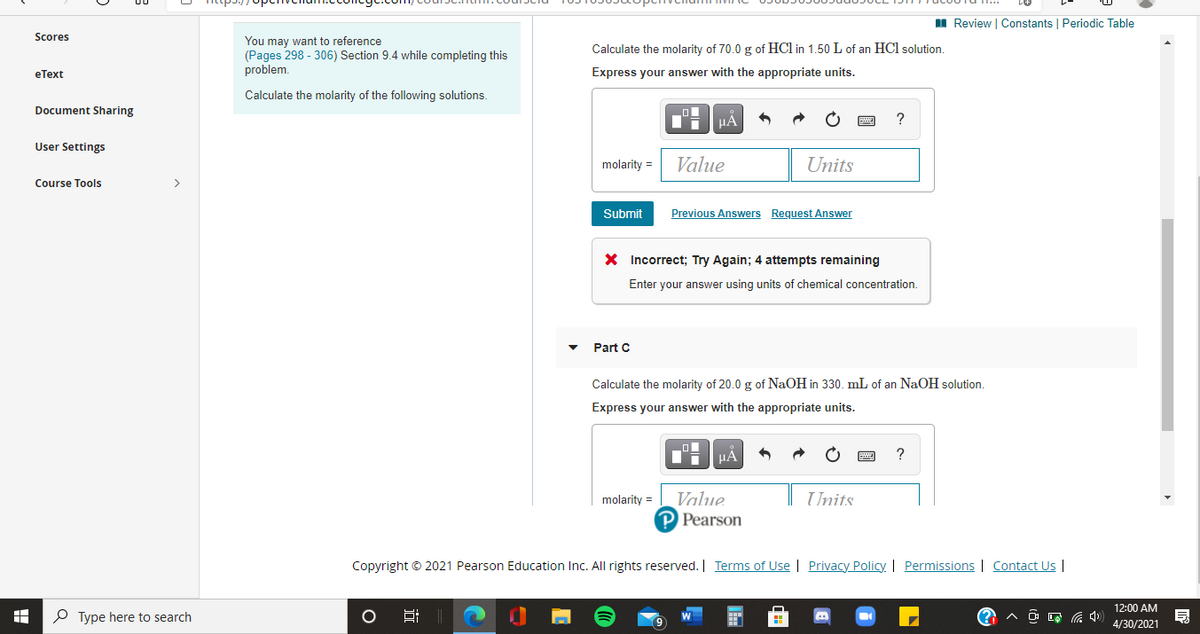
Calculate the molarity of 70.0 g of HCl in 1.50 L of an HCl solution.
eТеxt
Express your answer with the appropriate units.
Calculate the molarity of the following solutions.
Document Sharing
HẢ
?
User Settings
molarity =
Value
Units
Course Tools
>
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 4 attempts remaining
Enter your answer using units of chemical concentration.
Part C
Calculate the molarity of 20.0 g of NaOH in 330. mL of an NaOH solution.
Express your answer with the appropriate units.
?
molarity =
Value
Units
P Pearson
Copyright © 2021 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy. | Permissions | Contact Us |
12:00 AM
P Type here to search
司
4/30/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning