A solution is prepared at 25 °C that is initially 0.41 M in chloroacetic acid (HCH2CICO2), a weak acid with Ka = 1.3 × 103, and 1.3M in potassium chloroacetate (KCH2CICO2). Calculate the pH of the solution. Round your answer to 2 decimal places. pH = 5
A solution is prepared at 25 °C that is initially 0.41 M in chloroacetic acid (HCH2CICO2), a weak acid with Ka = 1.3 × 103, and 1.3M in potassium chloroacetate (KCH2CICO2). Calculate the pH of the solution. Round your answer to 2 decimal places. pH = 5
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter15: Solutions Of Acids And Bases
Section: Chapter Questions
Problem 15.121QE: A solution is made by diluting 25.0 mL of concentrated HCl (37% by weight; density = 1.19 g/mL) to...
Related questions
Question
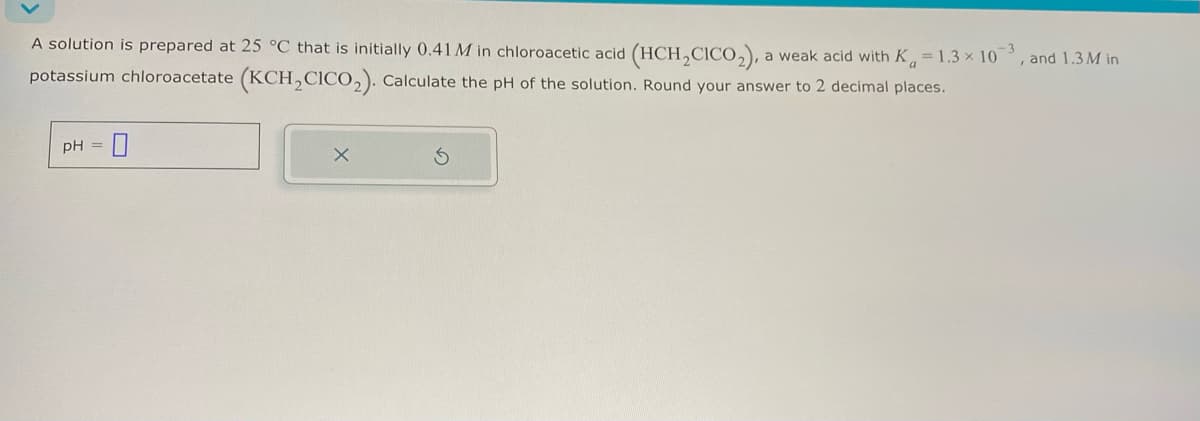
Transcribed Image Text:A solution is prepared at 25 °C that is initially 0.41 M in chloroacetic acid (HCH2CICO2), a weak acid with Ka = 1.3 × 103, and 1.3M in
potassium chloroacetate (KCH2CICO2). Calculate the pH of the solution. Round your answer to 2 decimal places.
pH =
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning