A stock solution whose concentration is 25.00 mg/mL was provided. A primary standard solution was then prepared by transferring 5.00 mL of the stock solution into a 50.00 mL volumetric flask. The primary standard solution was used to prepare three standard solutions of different concentrations by taking different volumes of aliquots into three 25.00 mL volumetric flasks: Standard Solution #1 #2 #3 Amount of Primary Solution (mL) 5.00 10.00 15.00 Three 25.00 mL volumetric flasks were then diluted to mark with water. Questions #1 What is the concentration of the primary standard solutions, expressed in units of mg/ml? #2 Re-write the concentration of the primary standard solutions obtained from Q#1 in unit of μg/mL. #3 Determine the concentrations of three standard solutions in units of µg/mL.
A stock solution whose concentration is 25.00 mg/mL was provided. A primary standard solution was then prepared by transferring 5.00 mL of the stock solution into a 50.00 mL volumetric flask. The primary standard solution was used to prepare three standard solutions of different concentrations by taking different volumes of aliquots into three 25.00 mL volumetric flasks: Standard Solution #1 #2 #3 Amount of Primary Solution (mL) 5.00 10.00 15.00 Three 25.00 mL volumetric flasks were then diluted to mark with water. Questions #1 What is the concentration of the primary standard solutions, expressed in units of mg/ml? #2 Re-write the concentration of the primary standard solutions obtained from Q#1 in unit of μg/mL. #3 Determine the concentrations of three standard solutions in units of µg/mL.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 49RGQ: Fluoridation of city water supplies has been practiced in the United States for several decades. It...
Related questions
Question
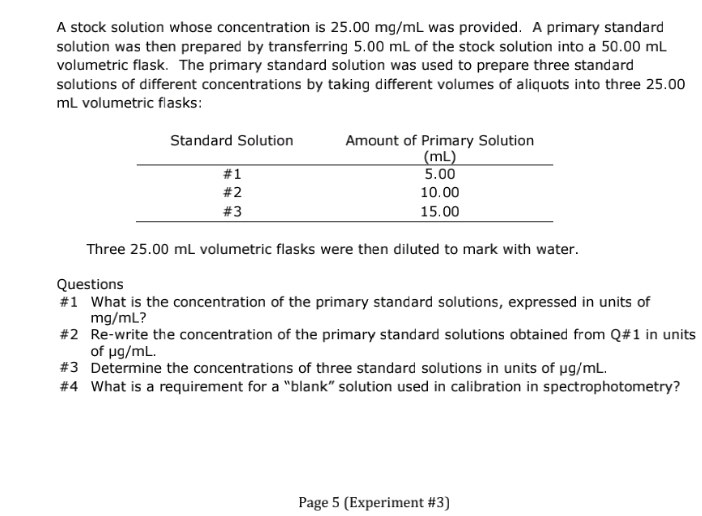
Transcribed Image Text:A stock solution whose concentration is 25.00 mg/mL was provided. A primary standard
solution was then prepared by transferring 5.00 mL of the stock solution into a 50.00 mL
volumetric flask. The primary standard solution was used to prepare three standard
solutions of different concentrations by taking different volumes of aliquots into three 25.00
mL volumetric flasks:
Standard Solution
#1
#2
#3
Amount of Primary Solution
(mL)
5.00
10.00
15.00
Three 25.00 mL volumetric flasks were then diluted to mark with water.
Questions
#1 What is the concentration of the primary standard solutions, expressed in units of
mg/ml?
#2
Re-write the concentration of the primary standard solutions obtained from Q#1 in units
of μg/mL.
#3 Determine the concentrations of three standard solutions in units of µg/mL.
#4 What is a requirement for a "blank" solution used in calibration in spectrophotometry?
Page 5 (Experiment #3)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning