A student decides to conduct an experiment by using two different flasks and two different gas samples. In flask 1, there exists Neon (Ne) gas, whereas the second flask is filled with nitrogen (N2) gas. If both flasks are kept at 300 K, answer the following questions. (Note: Molar mass of N2 = 28.014 g mol', %3D molar mass of Neon = 20.1797 g mol, R= 8.31 J. mol1.K-1, k= 1.38 x 10 -23 J.K-1, Avogadro's number = 6.02 x 1023 mol¯1.) а) Find the average kinetic energy of one Neon molecule. J b) Find the root-mean-square speed of one Neon molecule. ms-1
A student decides to conduct an experiment by using two different flasks and two different gas samples. In flask 1, there exists Neon (Ne) gas, whereas the second flask is filled with nitrogen (N2) gas. If both flasks are kept at 300 K, answer the following questions. (Note: Molar mass of N2 = 28.014 g mol', %3D molar mass of Neon = 20.1797 g mol, R= 8.31 J. mol1.K-1, k= 1.38 x 10 -23 J.K-1, Avogadro's number = 6.02 x 1023 mol¯1.) а) Find the average kinetic energy of one Neon molecule. J b) Find the root-mean-square speed of one Neon molecule. ms-1
Chapter2: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 81AP: One process for decaffeinating coffee uses carbon dioxide ( M=44.0 g/mol) at a molar density of...
Related questions
Question
Solve step by step and quick
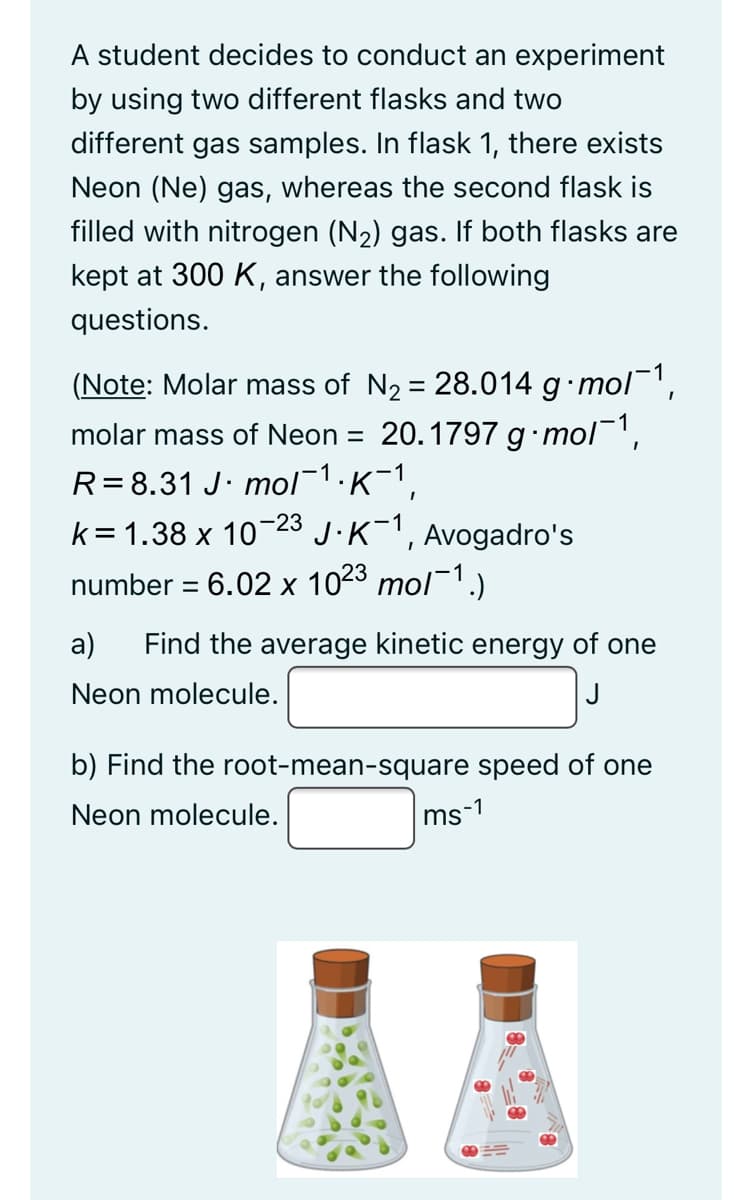
Transcribed Image Text:A student decides to conduct an experiment
by using two different flasks and two
different gas samples. In flask 1, there exists
Neon (Ne) gas, whereas the second flask is
filled with nitrogen (N2) gas. If both flasks are
kept at 300 K, answer the following
questions.
(Note: Molar mass of N2 = 28.014 g mol',
molar mass of Neon = 20.1797 g•mol,
R=8.31 J· mol1:K-1,
k= 1.38 x 1023 J.K-1,
number = 6.02 x 1023 mol¯1.)
Avogadro's
а)
Find the average kinetic energy of one
Neon molecule.
J
b) Find the root-mean-square speed of one
Neon molecule.
ms-1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning