A student made measurements on some electrochemical cells and calculated three quantities: • The standard reaction free energy AG". • The equilibrium constant K at 25.0 °C. • The cell potential under standard conditions E". 圖 His results are listed below. ol. Unfortunately, the student may have made some mistakes. Examine his results carefully and tick the box next to the incorrect quantity in each row, if any. Note: If there is a mistake in a row, only one of the three quantities listed is wrong. Also, you may assume the number of significant digits in each quantity is correct. Also note: for each cell, the number n of electrons transferred per redox reaction is 2. calculated quantities (Check the box next to any that are wrong.) cell n aG E K 174. kJ/mol 30 3.04 X 10 A -0.90 V 39 1.75 x 10 B 2 - 224. kJ/mol -1.16 V -33 8.71 x 10 2 - 183. kJ/mol -0.95 V
A student made measurements on some electrochemical cells and calculated three quantities: • The standard reaction free energy AG". • The equilibrium constant K at 25.0 °C. • The cell potential under standard conditions E". 圖 His results are listed below. ol. Unfortunately, the student may have made some mistakes. Examine his results carefully and tick the box next to the incorrect quantity in each row, if any. Note: If there is a mistake in a row, only one of the three quantities listed is wrong. Also, you may assume the number of significant digits in each quantity is correct. Also note: for each cell, the number n of electrons transferred per redox reaction is 2. calculated quantities (Check the box next to any that are wrong.) cell n aG E K 174. kJ/mol 30 3.04 X 10 A -0.90 V 39 1.75 x 10 B 2 - 224. kJ/mol -1.16 V -33 8.71 x 10 2 - 183. kJ/mol -0.95 V
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 22E: The mass of three different metal electrodes, each from a different galvanic cell, were determined...
Related questions
Question
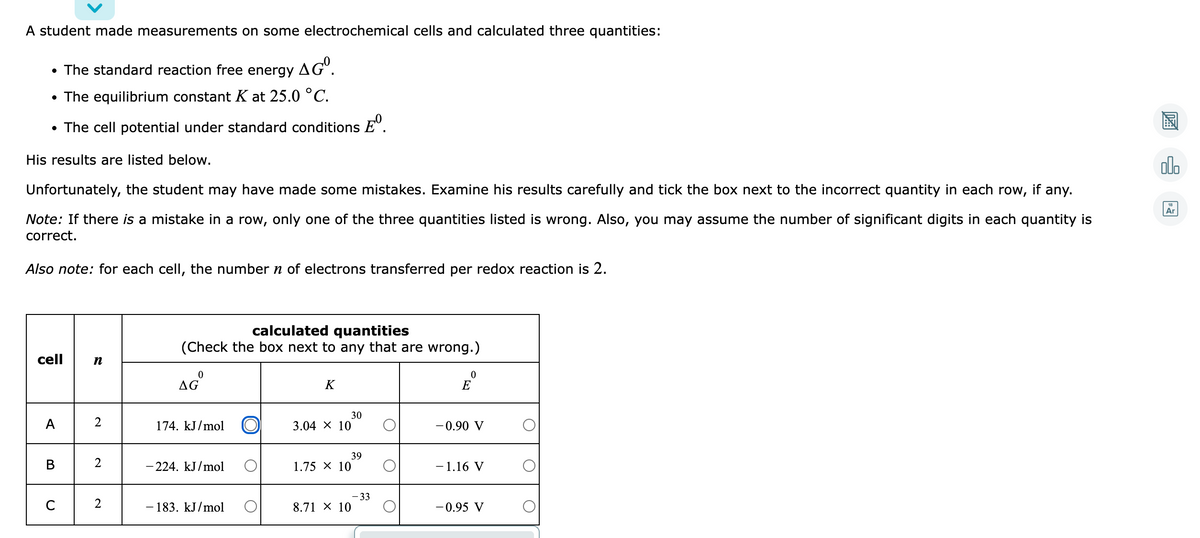
Transcribed Image Text:A student made measurements on some electrochemical cells and calculated three quantities:
• The standard reaction free energy
AGº.
• The equilibrium constant K at 25.0 °C.
• The cell potential under standard conditions E".
His results are listed below.
olo
Unfortunately, the student may have made some mistakes. Examine his results carefully and tick the box next to the incorrect quantity in each row, if any.
Ar
Note: If there is a mistake in a row, only one of the three quantities listed is wrong. Also, you
correct.
ay assume the number of significant digits in each quantity is
Also note: for each cell, the number n of electrons transferred per redox reaction is 2.
calculated quantities
(Check the box next to any that are wrong.)
cell
K
E
30
A
2
174. kJ/mol
3.04 X 10
-0.90 V
39
В
2
- 224. kJ/mol
1.75 X 10
- 1.16 V
- 33
8.71 X 10
C
2
183. kJ/mol
-0.95 V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning