A student titrates a 10-mL sample of vinegar with 0.50M sodium hydroxide (NaOH).The initial reading of the burette is 0.65 ml and the final reading is 9.20 mL. Calculate the mass percentage of acetic acid in the vinegar sample.(Molar mass of acetic acid%360 g/mol, density of vinegar=1.01g/mL). 4.02% ( 2.54% O 5.22% O 6.43%
A student titrates a 10-mL sample of vinegar with 0.50M sodium hydroxide (NaOH).The initial reading of the burette is 0.65 ml and the final reading is 9.20 mL. Calculate the mass percentage of acetic acid in the vinegar sample.(Molar mass of acetic acid%360 g/mol, density of vinegar=1.01g/mL). 4.02% ( 2.54% O 5.22% O 6.43%
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter4: Stoichiometry Of Chemical Reactions
Section: Chapter Questions
Problem 91E: A sample of solid calcium hydroxide, Ca(OH)2, is allowed to stand in water until a saturated...
Related questions
Question
Solve on paper in step
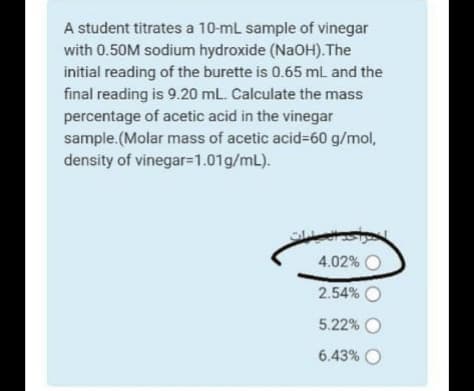
Transcribed Image Text:A student titrates a 10-mL sample of vinegar
with 0.50M sodium hydroxide (NaOH).The
initial reading of the burette is 0.65 mL and the
final reading is 9.20 mL. Calculate the mass
percentage of acetic acid in the vinegar
sample.(Molar mass of acetic acid%=60 g/mol,
density of vinegar=1.01g/mL).
4.02% O
2.54% O
5.22% O
6.43% O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning