A student was given an unknown liquid and told that it is one of the liquids in the box below. He weighed a 50-mL graduated cylinder then he poured 20.0 mL of the liquid in it and weighed it again. He recorded the following masses in his notebook: Mass of 50-mL graduated cylinder 59.3876 g Mass of 50-ml graduated cylinder + liquid 89.2876 g Chemical Density (g/mL) 1.027 chlorobenzene 1,3-dichloropropane n-butyl bromide 1,2,3-trichlorbenzene chloroform carbon tetrachloride 1.189 1.299 1.417 1.498 1.595 What is the likely identity of the liquid? Carbon tetrachloride 1,2,3-trichlorobenzene n-butyl bromide Chloroform
A student was given an unknown liquid and told that it is one of the liquids in the box below. He weighed a 50-mL graduated cylinder then he poured 20.0 mL of the liquid in it and weighed it again. He recorded the following masses in his notebook: Mass of 50-mL graduated cylinder 59.3876 g Mass of 50-ml graduated cylinder + liquid 89.2876 g Chemical Density (g/mL) 1.027 chlorobenzene 1,3-dichloropropane n-butyl bromide 1,2,3-trichlorbenzene chloroform carbon tetrachloride 1.189 1.299 1.417 1.498 1.595 What is the likely identity of the liquid? Carbon tetrachloride 1,2,3-trichlorobenzene n-butyl bromide Chloroform
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 56RGQ: You are asked to identify an unknown liquid that is known to be one of the liquids listed below. You...
Related questions
Question
Answer these
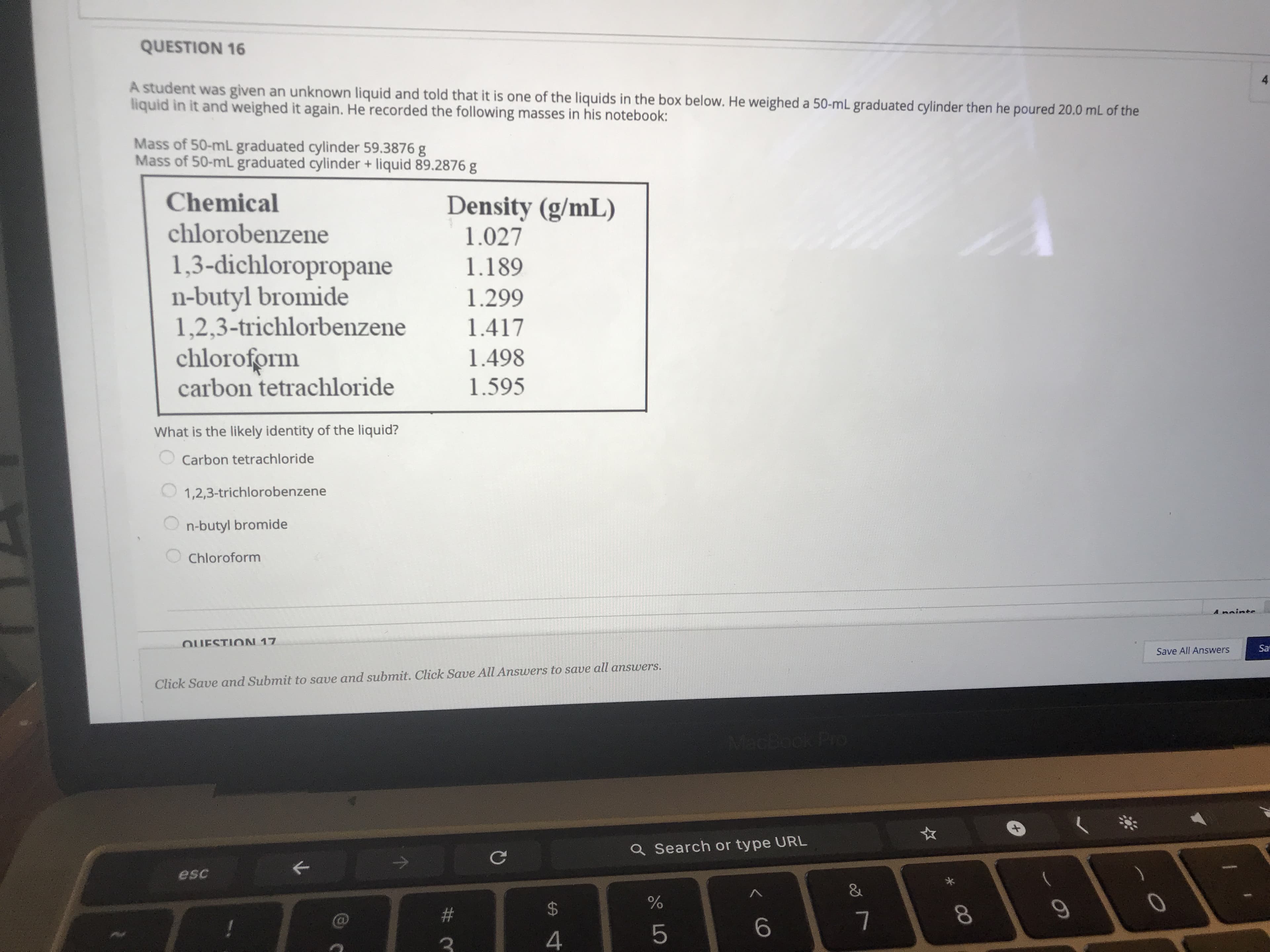
Transcribed Image Text:A student was given an unknown liquid and told that it is one of the liquids in the box below. He weighed a 50-mL graduated cylinder then he poured 20.0 mL of the
liquid in it and weighed it again. He recorded the following masses in his notebook:
Mass of 50-mL graduated cylinder 59.3876 g
Mass of 50-ml graduated cylinder + liquid 89.2876 g
Chemical
Density (g/mL)
1.027
chlorobenzene
1,3-dichloropropane
n-butyl bromide
1,2,3-trichlorbenzene
chloroform
carbon tetrachloride
1.189
1.299
1.417
1.498
1.595
What is the likely identity of the liquid?
Carbon tetrachloride
1,2,3-trichlorobenzene
n-butyl bromide
Chloroform
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning