Consider the following compounds and their densities. Density Density Substance (g/mL) Substance (9/mL) Isopropyl alcohol 0.785 Toluene 0.866 n-Butyl alcohol 0.810 Ethylene glycol 1.114 You create a column of the liquids in a glass cylinder with the most dense material on the bottom layer and the least dense on the top. You do not allow the liquids to mix. a First you drop a plastic bead that has a density of 0.32 g/cm° into the column. What do you expect to observe? The bead will float on top of all the liquids. The bead will pass through the top layer and float on the n-butyl alcohol layer. The bead will pass through the top two layers and float on the toluene layer. The bead will pass through the top three layers and float on the ethylene glycol layer. The bead will sink all the way to the bottom. Correct Since the bead is less dense than any of the liquids in the column, the bead will float on top of all the liquids. b Next you drop a different plastic bead that has a volume of 0.058 mL and a mass of 5.22 x 10- g into the column. What would you expect to observe in this case? O The bead will float on top of all the liquids. O The bead will pass through the top layer and float on the n-butyl alcohol layer. O The bead will pass through the top two layers and float on the toluene layer. O The bead will pass through the top three layers and float on the ethylene glycol layer. O The bead will sink all the way to the bottom.
Consider the following compounds and their densities. Density Density Substance (g/mL) Substance (9/mL) Isopropyl alcohol 0.785 Toluene 0.866 n-Butyl alcohol 0.810 Ethylene glycol 1.114 You create a column of the liquids in a glass cylinder with the most dense material on the bottom layer and the least dense on the top. You do not allow the liquids to mix. a First you drop a plastic bead that has a density of 0.32 g/cm° into the column. What do you expect to observe? The bead will float on top of all the liquids. The bead will pass through the top layer and float on the n-butyl alcohol layer. The bead will pass through the top two layers and float on the toluene layer. The bead will pass through the top three layers and float on the ethylene glycol layer. The bead will sink all the way to the bottom. Correct Since the bead is less dense than any of the liquids in the column, the bead will float on top of all the liquids. b Next you drop a different plastic bead that has a volume of 0.058 mL and a mass of 5.22 x 10- g into the column. What would you expect to observe in this case? O The bead will float on top of all the liquids. O The bead will pass through the top layer and float on the n-butyl alcohol layer. O The bead will pass through the top two layers and float on the toluene layer. O The bead will pass through the top three layers and float on the ethylene glycol layer. O The bead will sink all the way to the bottom.
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter2: Matter And Energy
Section: Chapter Questions
Problem 2.4TC: Specific gravity is a physical property. Beakers hold three clear, colorless liquids A, B, and C....
Related questions
Question
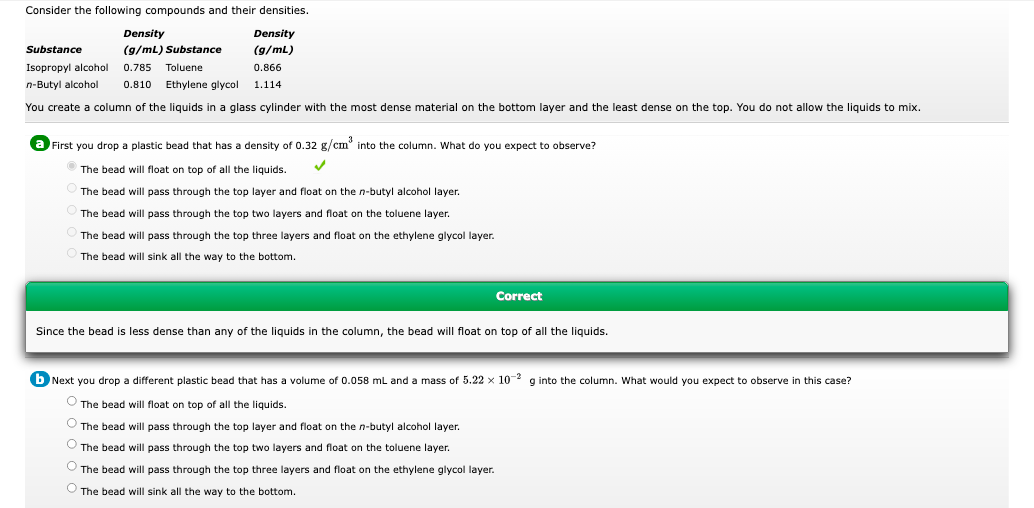
Transcribed Image Text:Consider the following compounds and their densities.
Density
Density
Substance
(g/mL) Substance
(9/mL)
Isopropyl alcohol
0.785 Toluene
0.866
n-Butyl alcohol
0.810
Ethylene glycol
1.114
You create a column of the liquids in a glass cylinder with the most dense material on the bottom layer and the least dense on the top. You do not allow the liquids to mix.
a First you drop a plastic bead that has a density of 0.32 g/cm° into the column. What do you expect to observe?
The bead will float on top of all the liquids.
The bead will pass through the top layer and float on the n-butyl alcohol layer.
The bead will pass through the top two layers and float on the toluene layer.
The bead will pass through the top three layers and float on the ethylene glycol layer.
The bead will sink all the way to the bottom.
Correct
Since the bead is less dense than any of the liquids in the column, the bead will float on top of all the liquids.
b Next you drop a different plastic bead that has a volume of 0.058 mL and a mass of 5.22 x 10- g into the column. What would you expect to observe in this case?
O The bead will float on top of all the liquids.
O The bead will pass through the top layer and float on the n-butyl alcohol layer.
O The bead will pass through the top two layers and float on the toluene layer.
O The bead will pass through the top three layers and float on the ethylene glycol layer.
O The bead will sink all the way to the bottom.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning