A student weighed their penny (mass 2.499 g) and followed the same experimental procedure you did in the lab. They found that the penny contained 0.0980 g of copper, or, 3.92%. Estimate the thickness of the copper coating with the following formula. From the US Mint website: the diameter of a penny is 19.05 mm and the thickness is 1.52 mm. The density of copper is 8.96 g/cm3. TIP: your final answer will have the units of cm (think about what to do first..). mass of Cu/ volume density of Cu Thickness of Cu Coating = area diameter + T(diameter) (thickness)
A student weighed their penny (mass 2.499 g) and followed the same experimental procedure you did in the lab. They found that the penny contained 0.0980 g of copper, or, 3.92%. Estimate the thickness of the copper coating with the following formula. From the US Mint website: the diameter of a penny is 19.05 mm and the thickness is 1.52 mm. The density of copper is 8.96 g/cm3. TIP: your final answer will have the units of cm (think about what to do first..). mass of Cu/ volume density of Cu Thickness of Cu Coating = area diameter + T(diameter) (thickness)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter1: The Nature Of Chemistry
Section: Chapter Questions
Problem 116QRT
Related questions
Question
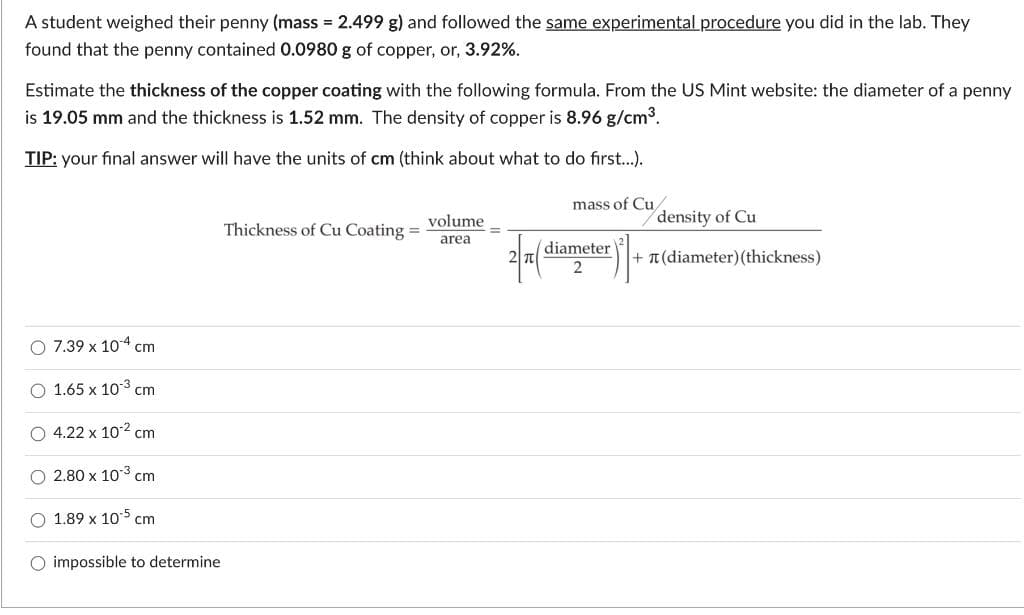
Transcribed Image Text:A student weighed their penny (mass = 2.499 g) and followed the same experimental procedure you did in the lab. They
found that the penny contained 0.0980 g of copper, or, 3.92%.
Estimate the thickness of the copper coating with the following formula. From the US Mint website: the diameter of a penny
is 19.05 mm and the thickness is 1.52 mm. The density of copper is 8.96 g/cm3.
TIP: your final answer will have the units of cm (think about what to do first.).
mass of Cu
volume
density of Cu
Thickness of Cu Coating =
area
(diameter
2 T
T (diameter) (thickness)
O 7.39 x 104 cm
O 1.65 x 103 cm
O 4.22 x 102 cm
O 2.80 x 103 cm
O 1.89 x 105 cm
O impossible to determine
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning