a) The structure of the compound b) The molecular ion (M*) and its corresponding m/z value (if shown in the spectrum) c) The base peak and its corresponding m/z value d) Provide the fragmentation pattern of the molecule. Be sure to clearly indicate the cations and/or radical fragments and their corresponding m/z value from the spectrum. Compound Name: Methyl propionate 120 100 57 80 60 20
a) The structure of the compound b) The molecular ion (M*) and its corresponding m/z value (if shown in the spectrum) c) The base peak and its corresponding m/z value d) Provide the fragmentation pattern of the molecule. Be sure to clearly indicate the cations and/or radical fragments and their corresponding m/z value from the spectrum. Compound Name: Methyl propionate 120 100 57 80 60 20
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
ChapterL2: Mass Spectrometry
Section: Chapter Questions
Problem 3E
Related questions
Question
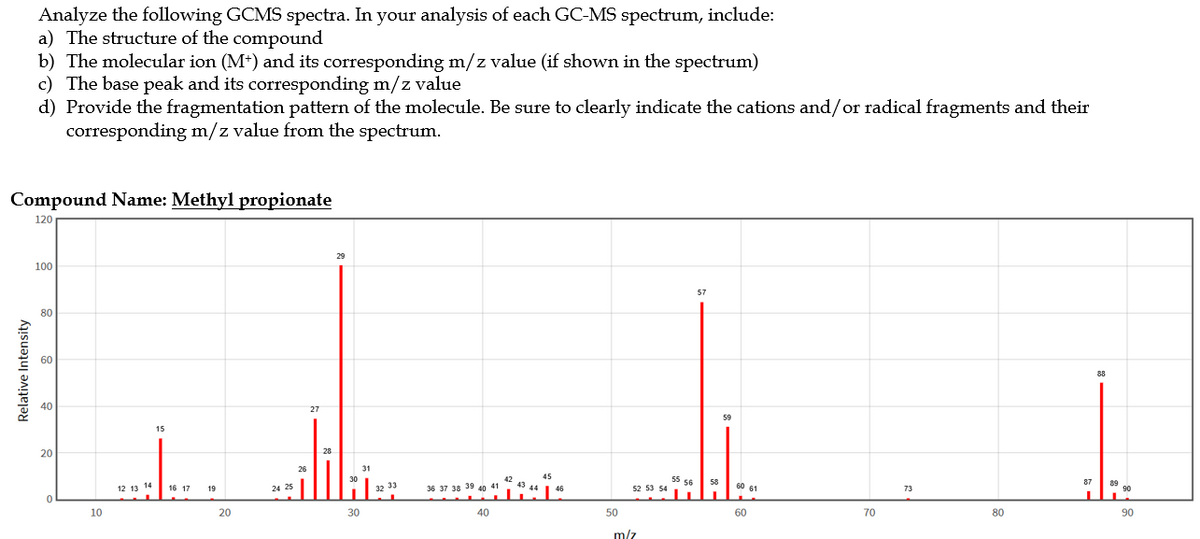
Transcribed Image Text:Analyze the following GCMS spectra. In your analysis of each GC-MS spectrum, include:
a) The structure of the compound
b) The molecular ion (M*) and its corresponding m/z value (if shown in the spectrum)
c) The base peak and its corresponding m/z value
d) Provide the fragmentation pattern of the molecule. Be sure to clearly indicate the cations and/or radical fragments and their
corresponding m/z value from the spectrum.
Compound Name: Methyl propionate
120
100
57
80
40
59
15
20
87
12 13 14
52 53 54
60 61
89 90
19
36 37 38
73
10
20
30
40
50
60
70
80
90
m/z
Relative Intensity
Expert Solution
Step 1
Mass spectra is used to identify the mass of the compound and to know the mass fragmentation pattern of the compound.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole