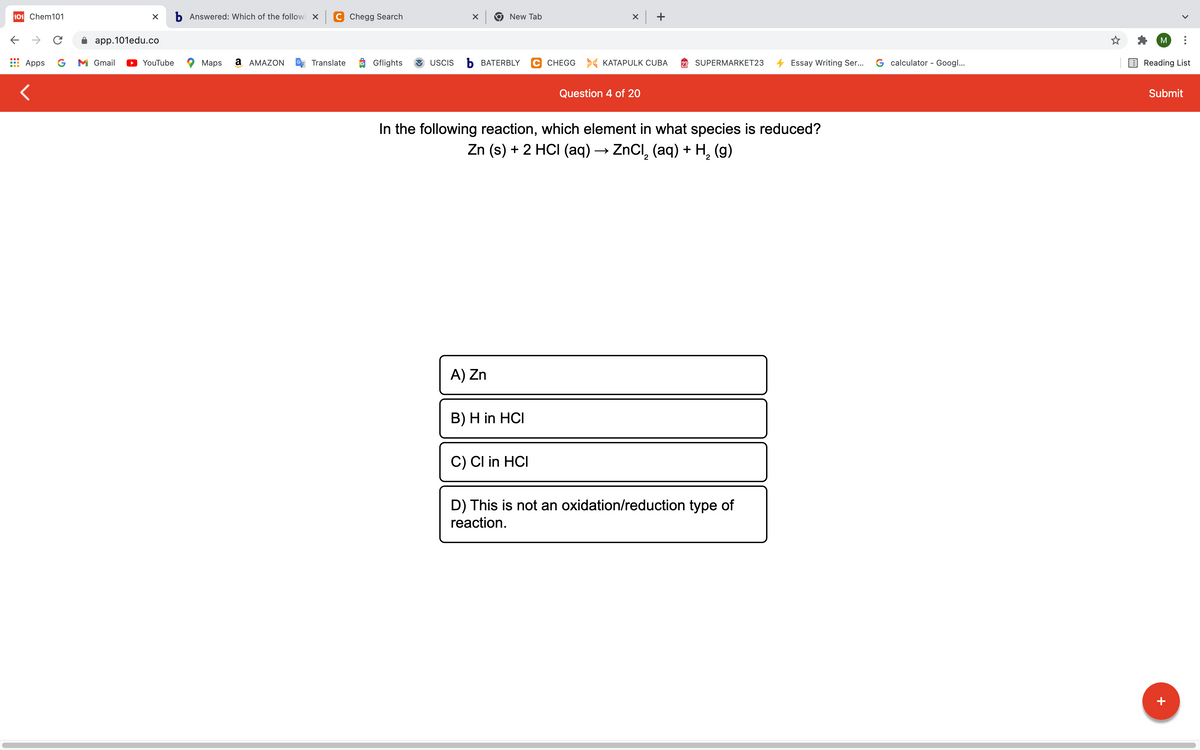
A Translate a oflights 3 USCIS BATERBLY G CHEGG KATAPULK CUBA SUPERMARKET23 4 Essay Writing Ser G calculator - Googl Question 4 of 20 In the following reaction, which element in what species is reduced? Zn (s) + 2 HCI (aq) → ZNCI, (aq) + H, (9) A) Zn B) H in HCI C) Cl in HCI D) This is not an oxidation/reduction type of reaction.
A Translate a oflights 3 USCIS BATERBLY G CHEGG KATAPULK CUBA SUPERMARKET23 4 Essay Writing Ser G calculator - Googl Question 4 of 20 In the following reaction, which element in what species is reduced? Zn (s) + 2 HCI (aq) → ZNCI, (aq) + H, (9) A) Zn B) H in HCI C) Cl in HCI D) This is not an oxidation/reduction type of reaction.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter4: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 10P: Use the data in Figure 4.8 to estimate the ratio of radiation intensity at 10,000 Å (infrared) to...
Related questions
Question
Transcribed Image Text:101 Chem101
b Answered: Which of the followi X C Chegg Search
9 New Tab
->
app.101edu.co
M
Apps
G
M Gmail
YouTube
Maps
а АМAZON
Translate
Gflights
USCIS
Ь ВАТERBLY
C CHEGG > KATAPULK CUBA
SUPERMARKET23
Essay Writing Ser...
G calculator - Googl...
Reading List
Question 4 of 20
Submit
In the following reaction, which element in what species is reduced?
Zn (s) + 2 HCI (aq) → ZnCI, (aq) + H, (g)
A) Zn
B) H in HCI
C) CI in HCI
D) This is not an oxidation/reduction type of
reaction.
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning