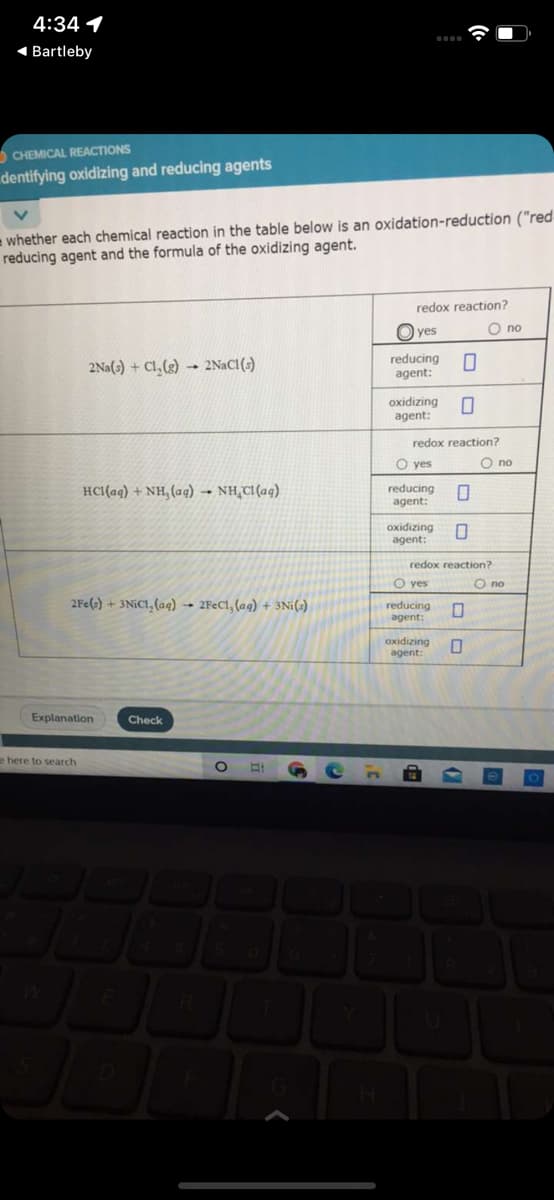
= whether each chemical reaction in the table below is an oxidation-reduction ("red reducing agent and the formula of the oxidizing agent. redox reaction? yes O no 2Na(-) + C1,(2) 2NACI() reducing O agent: oxidizing agent: redox reaction? O yes O no HCI(aq) + NH, (aq) → NH,CI(aq) reducing agent: oxidizing agent: redox reaction? O yes O no 2Pe()+ 3NIC1, (aq) 2FEC1, (ag) + 3Ni() reducing agent: oxidizing agent:
= whether each chemical reaction in the table below is an oxidation-reduction ("red reducing agent and the formula of the oxidizing agent. redox reaction? yes O no 2Na(-) + C1,(2) 2NACI() reducing O agent: oxidizing agent: redox reaction? O yes O no HCI(aq) + NH, (aq) → NH,CI(aq) reducing agent: oxidizing agent: redox reaction? O yes O no 2Pe()+ 3NIC1, (aq) 2FEC1, (ag) + 3Ni() reducing agent: oxidizing agent:
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter4: Chemical Reactions
Section: Chapter Questions
Problem 4.92P: 4-92 Reaction of pentane, C5H12, with oxygen, O2, gives carbon dioxide and water. (a) Write a...
Related questions
Question
Transcribed Image Text:4:34 1
1 Bartleby
) CHEMICAL REACTIONS
dentifying oxidizing and reducing agents
e whether each chemical reaction in the table below is an oxidation-reduction ("red
reducing agent and the formula of the oxidizing agent.
redox reaction?
O yes
О по
2Na(s) + Cl, (g) 2NACI(s)
reducing
agent:
oxidizing
agent:
redox reaction?
O yes
HCI(aq) + NH, (aq) → NH,CI (aq)
reducing
agent:
oxidizing
agent:
redox reaction?
O yes
O no
2Fe(s) + 3NIC1, (aq) → 2F€C1, (ag) + 3Ni(s)
reducing
agent:
agent:
Buizipixo
Explanation
Check
e here to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning
