(a) Use Bohr's model of the hydrogen atom to show that when the electron moves from the n state to the n – 1 state, the frequency of the emitted light is 2n – 1 e f = п?(п — 1)2 (b) Bohr's correspondence principle claims that quantum results should reduce to classical results in the limit of large quantum numbers. Show that as n→ 0, this expres- sion varies as 1/n³ and reduces to the classical frequency one expects the atom to emit. Suggestion: To calculate the classical frequency, note that the frequency of revolution is v/2Tr, where vis the speed of the electron and ris given by Equation 41.10. n²h? ke? n?h? n = 1, 2, 3, . .. (41.10) mr m,k_e? e"e
(a) Use Bohr's model of the hydrogen atom to show that when the electron moves from the n state to the n – 1 state, the frequency of the emitted light is 2n – 1 e f = п?(п — 1)2 (b) Bohr's correspondence principle claims that quantum results should reduce to classical results in the limit of large quantum numbers. Show that as n→ 0, this expres- sion varies as 1/n³ and reduces to the classical frequency one expects the atom to emit. Suggestion: To calculate the classical frequency, note that the frequency of revolution is v/2Tr, where vis the speed of the electron and ris given by Equation 41.10. n²h? ke? n?h? n = 1, 2, 3, . .. (41.10) mr m,k_e? e"e
Modern Physics
3rd Edition
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Chapter4: The Particle Nature Of Matter
Section: Chapter Questions
Problem 37P
Related questions
Question
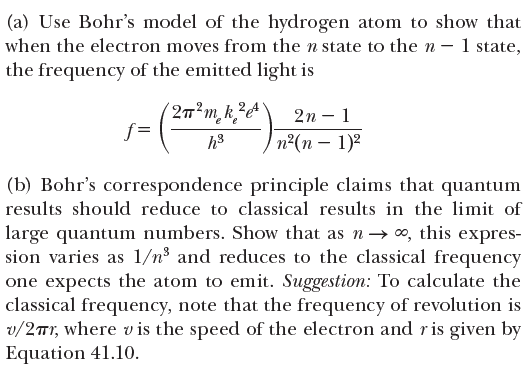
Transcribed Image Text:(a) Use Bohr's model of the hydrogen atom to show that
when the electron moves from the n state to the n – 1 state,
the frequency of the emitted light is
2n – 1
e
f =
п?(п — 1)2
(b) Bohr's correspondence principle claims that quantum
results should reduce to classical results in the limit of
large quantum numbers. Show that as n→ 0, this expres-
sion varies as 1/n³ and reduces to the classical frequency
one expects the atom to emit. Suggestion: To calculate the
classical frequency, note that the frequency of revolution is
v/2Tr, where vis the speed of the electron and ris given by
Equation 41.10.
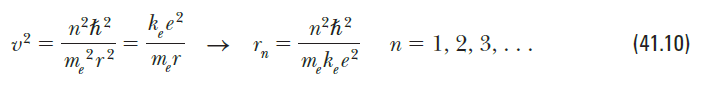
Transcribed Image Text:n²h?
ke?
n?h?
n = 1, 2, 3, . ..
(41.10)
mr
m,k_e?
e"e
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 7 images

Recommended textbooks for you

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax