In the X-ray spectrum of the element with Z= 43, a Ka peak is observed at a wavelength of 6.718 x 1011 m. (a) Determine the difference between the observed wavelength of the K, X-ray for this element and that predicted by the Bohr model. (b) Express this difference (the absolute value) as a percentage of the observed wavelength. K shell L shell Bohr-model picture of innermost shells of target atom (a) Number 0.00000000 586 Units m (b) Numberi 1.958 Units percent
In the X-ray spectrum of the element with Z= 43, a Ka peak is observed at a wavelength of 6.718 x 1011 m. (a) Determine the difference between the observed wavelength of the K, X-ray for this element and that predicted by the Bohr model. (b) Express this difference (the absolute value) as a percentage of the observed wavelength. K shell L shell Bohr-model picture of innermost shells of target atom (a) Number 0.00000000 586 Units m (b) Numberi 1.958 Units percent
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter28: Quantum Physics
Section: Chapter Questions
Problem 27P
Related questions
Question
Physics
please provide correct answer with unit and significan digits.
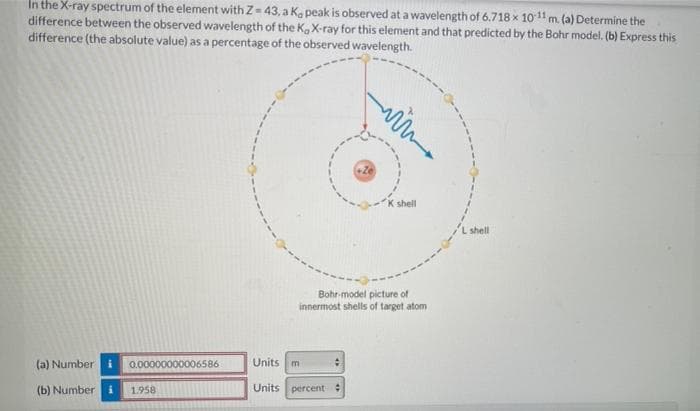
Transcribed Image Text:In the X-ray spectrum of the element with Z= 43, a Ka peak is observed at a wavelength of 6.718 x 1011 m. (a) Determine the
difference between the observed wavelength of the Ka X-ray for this element and that predicted by the Bohr model. (b) Express this
difference (the absolute value) as a percentage of the observed wavelength.
K shell
L shell
Bohr-model picture of
innermost shells of target atom
(a) Number
0.00000000
Units m
(b) Number
1.958
Units percent:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax