A) Use the Law of Definite Proportions to describe the relationship between the three compounds shown in the image above: NO, NO2, and N2O. B) Use the Law of Multiple Proportions to describe the relationship between the three compounds shown in the image above: NO, NO2, and N2O.
A) Use the Law of Definite Proportions to describe the relationship between the three compounds shown in the image above: NO, NO2, and N2O. B) Use the Law of Multiple Proportions to describe the relationship between the three compounds shown in the image above: NO, NO2, and N2O.
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter3: Matter-properties And Changes
Section3.4: Elements And Compounds
Problem 28SSC
Related questions
Question
A) Use the Law of Definite Proportions to describe the relationship between the three compounds shown in the image above: NO, NO2, and N2O.
B) Use the Law of Multiple Proportions to describe the relationship between the three compounds shown in the image above: NO, NO2, and N2O.
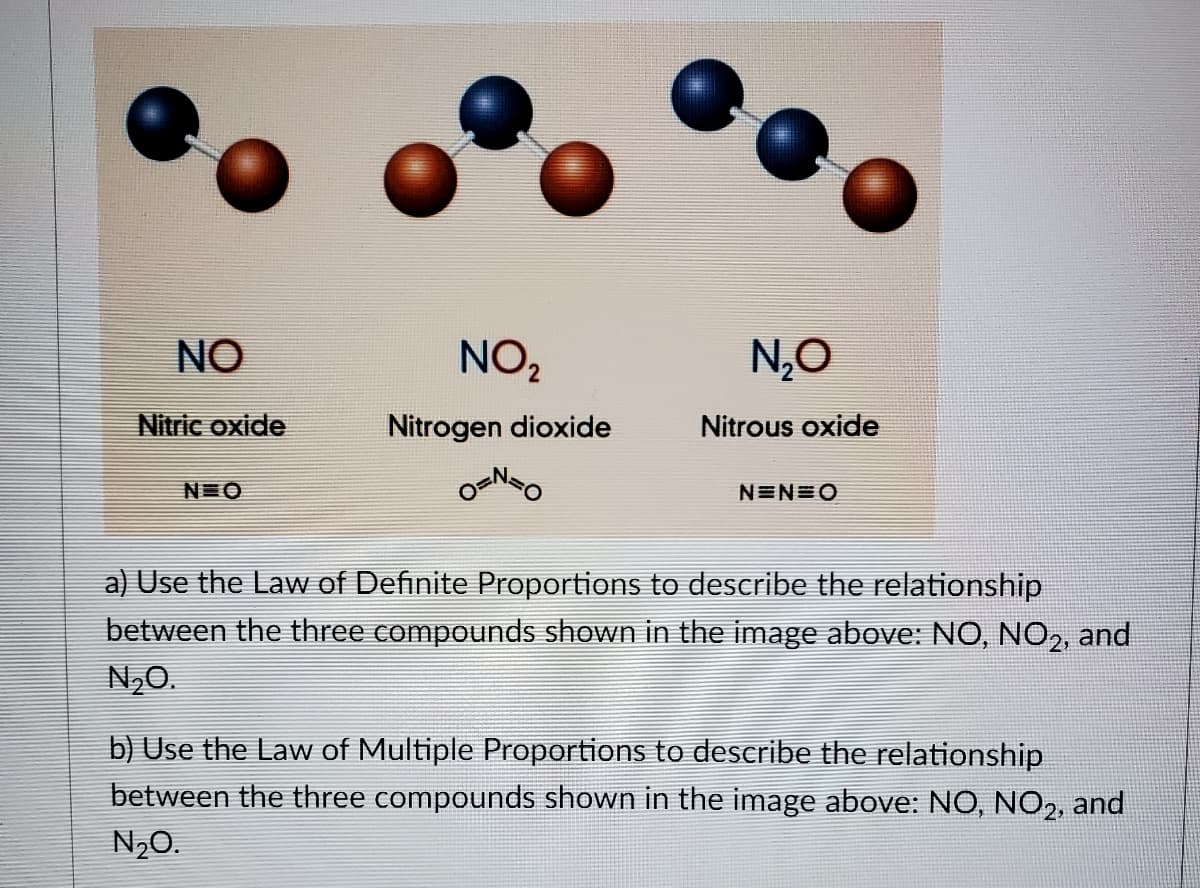
Transcribed Image Text:NO
NO,
N,O
Nitric oxide
Nitrogen dioxide
Nitrous oxide
N O
NEN=O
a) Use the Law of Definite Proportions to describe the relationship
between the three compounds shown in the image above: NO, NO,, and
N,0.
b) Use the Law of Multiple Proportions to describe the relationship
between the three compounds shown in the image above: NO, NO2, and
N20.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning