4. Which pair could be used to demonstrate the law of multiple proportions? O A. CuCl2 and CuCl O B. MgS and MgCl O C. KF and CaF2 O D. NaCl and KCI
4. Which pair could be used to demonstrate the law of multiple proportions? O A. CuCl2 and CuCl O B. MgS and MgCl O C. KF and CaF2 O D. NaCl and KCI
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter2: Chemical Formulas, Equations, And Reaction Yields
Section: Chapter Questions
Problem 17P: A gaseous binary compound has a vapor density that is 1.94 times that of oxygen at the same...
Related questions
Question
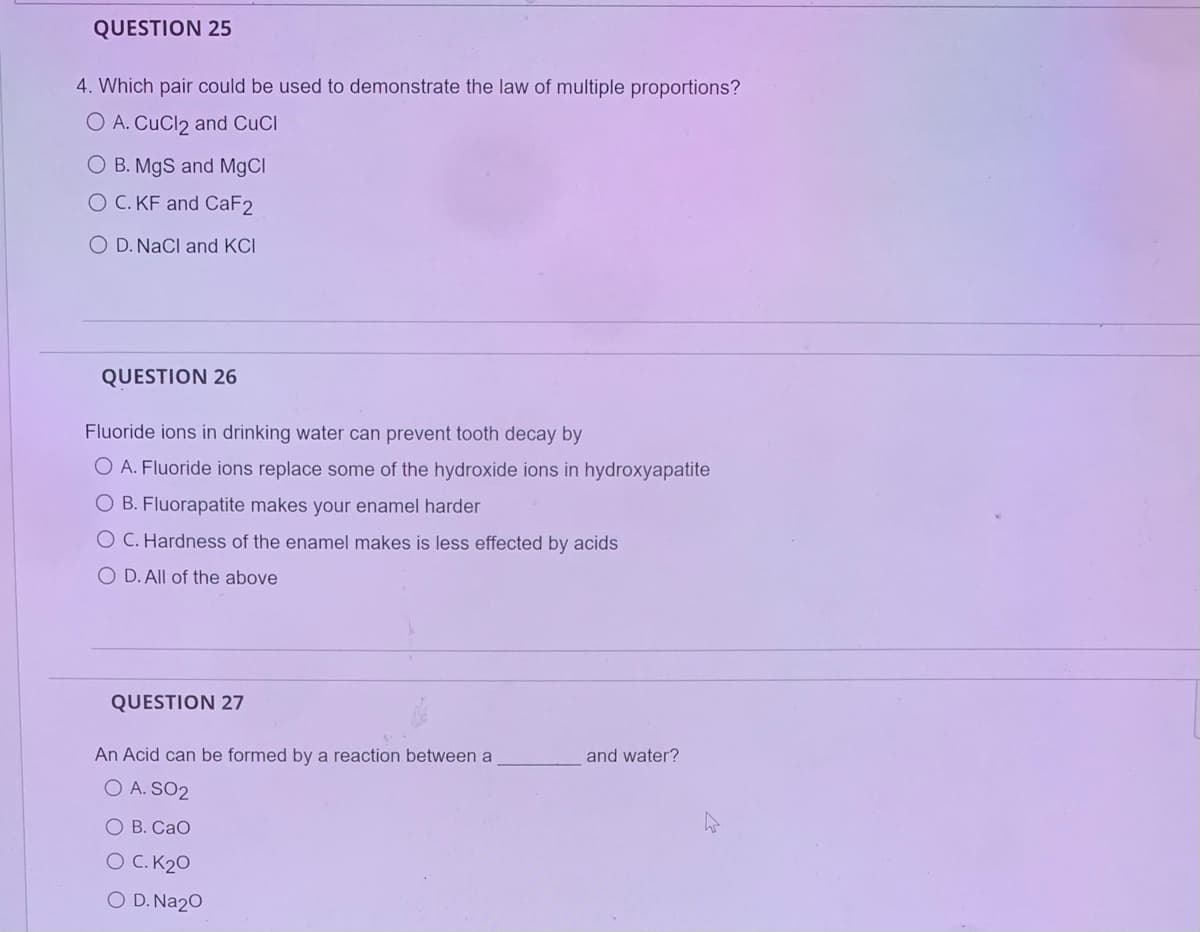
Transcribed Image Text:QUESTION 25
4. Which pair could be used to demonstrate the law of multiple proportions?
O A. CuCl2 and CuCl
O B. MgS and MgCl
O C. KF and CaF2
O D. NaCl and KCI
QUESTION 26
Fluoride ions in drinking water can prevent tooth decay by
O A. Fluoride ions replace some of the hydroxide ions in hydroxyapatite
O B. Fluorapatite makes your enamel harder
O C. Hardness of the enamel makes is less effected by acids
O D. All of the above
QUESTION 27
An Acid can be formed by a reaction between a
O A. SO2
O B. CaO
O C. K₂0
O D. Na₂O
and water?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning