A voltaic cell is constructed from a standard Fe2" Fe half cell (E°red = -0.440V) and a standard Mg*|Mg half cell (E°red = -2.370V). The anode reaction is: The cathode reaction is: The spontaneous cell reaction is: The cell voltage is V. +
A voltaic cell is constructed from a standard Fe2" Fe half cell (E°red = -0.440V) and a standard Mg*|Mg half cell (E°red = -2.370V). The anode reaction is: The cathode reaction is: The spontaneous cell reaction is: The cell voltage is V. +
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter8: Electrochemistry And Ionic Solutions
Section: Chapter Questions
Problem 8.11E
Related questions
Question
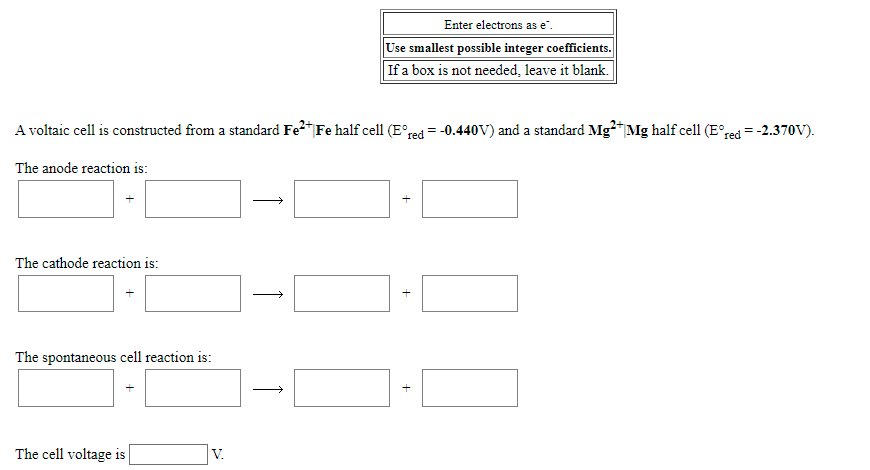
Transcribed Image Text:Enter electrons as e".
Use smallest possible integer coefficients.
If a box is not needed, leave it blank.
A voltaic cell is constructed from a standard Fe2"Fe half cell (E°red = -0.440V) and a standard Mg?*Mg half cell (E°red =-2.370V).
The anode reaction is:
The cathode reaction is:
The spontaneous cell reaction is:
The cell voltage is
V.
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning