A voltaic cell is constructed in which the anode is a III, half cell and the cathode is a FF, half cell. The half-cell compartments are connected by a salt bridge. (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank. The anode reaction is: The cathode reaction is: + The net cell reaction is: In the external circuit, electrons migrate the FIF₂ electrode ✓the I 1₂ electrode. In the salt bridge, anions migrate ✓the II₂ compartment. the FIF₂ compartment
A voltaic cell is constructed in which the anode is a III, half cell and the cathode is a FF, half cell. The half-cell compartments are connected by a salt bridge. (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank. The anode reaction is: The cathode reaction is: + The net cell reaction is: In the external circuit, electrons migrate the FIF₂ electrode ✓the I 1₂ electrode. In the salt bridge, anions migrate ✓the II₂ compartment. the FIF₂ compartment
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 14P
Related questions
Question
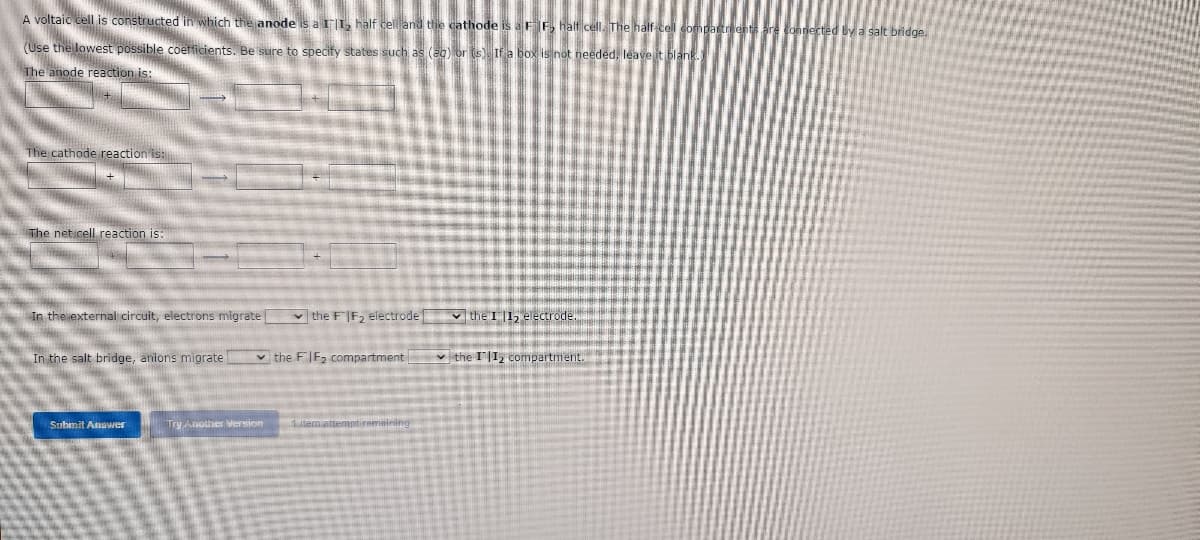
Transcribed Image Text:A voltaic cell is constructed in which the anode is a II, half cell and the cathode is a FF₂ half cell. The half-cell comp
(Use the lowest possible coefficients. Be sure to specify states such as (ag) or (s). If a box is not needed, leave it blank.
The anode reaction is:
The cathode reaction is:
The net cell reaction is:
In the external circuit, electrons migrate
✓the FIF₂ electrode the I 11₂ electrode.
In the salt bridge, anions migrate
the FIF₂ compartment
✓the II₂ compartment.
Submit Answer
pt remaining
ry Another version
by a salt bridge.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning