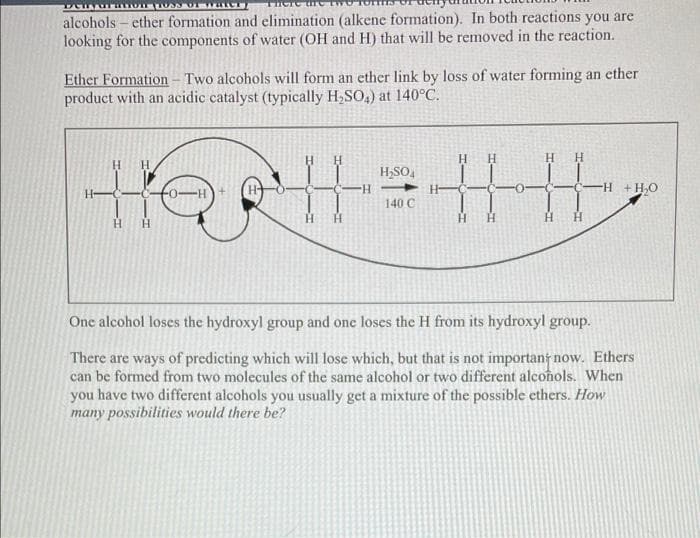
Ether Formation - Two alcohols will form an ether link by loss of water forming an ether product with an acidic catalyst (typically H₂SO4) at 140°C. H₂SO4 4.4 4 H-H 140 C One alcohol loses the hydroxyl group and one loses the H from its hydroxyl group. There are ways of predicting which will lose which, but that is not important now. Ethers can be formed from two molecules of the same alcohol or two different alcohols. When you have two different alcohols you usually get a mixture of the possible ethers. How many possibilities would there be? -H + H₂O
Ether Formation - Two alcohols will form an ether link by loss of water forming an ether product with an acidic catalyst (typically H₂SO4) at 140°C. H₂SO4 4.4 4 H-H 140 C One alcohol loses the hydroxyl group and one loses the H from its hydroxyl group. There are ways of predicting which will lose which, but that is not important now. Ethers can be formed from two molecules of the same alcohol or two different alcohols. When you have two different alcohols you usually get a mixture of the possible ethers. How many possibilities would there be? -H + H₂O
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 52E: Before the introduction of chlorofluorocarbons, sulfur dioxide (enthalpy of vaporization, 6.00...
Related questions
Question
Transcribed Image Text:THOTC
alcoholsether formation and elimination (alkene formation). In both reactions you are
looking for the components of water (OH and H) that will be removed in the reaction.
Ether Formation - Two alcohols will form an ether link by loss of water forming an ether
product with an acidic catalyst (typically H₂SO4) at 140°C.
Н Н
Η Η
H₂SO4
-H
-H + H₂O
HellH|4
140 C
Н
One alcohol loses the hydroxyl group and one loses the H from its hydroxyl group.
There are ways of predicting which will lose which, but that is not important now. Ethers
can be formed from two molecules of the same alcohol or two different alcohols. When
you have two different alcohols you usually get a mixture of the possible ethers. How
many possibilities would there be?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax