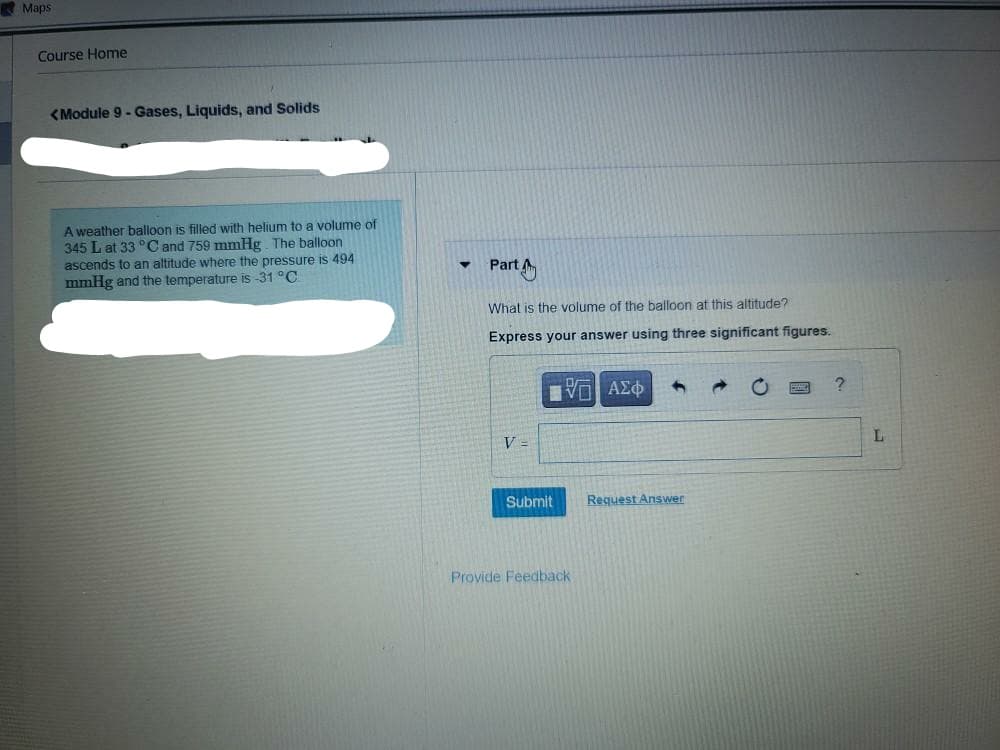
A weather balloon is filled with helium to a volume of 345 L at 33 °C and 759 mmHg The balloon ascends to an altitude where the pressure is 494 mmHg and the temperature is -31 °C Part What is the volume of the balloon at this aititude? Express your answer using three significant figures.
A weather balloon is filled with helium to a volume of 345 L at 33 °C and 759 mmHg The balloon ascends to an altitude where the pressure is 494 mmHg and the temperature is -31 °C Part What is the volume of the balloon at this aititude? Express your answer using three significant figures.
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 25A
Related questions
Question
Transcribed Image Text:R Maps
Course Home
<Module 9 - Gases, Liquids, and Solids
A weather balloon is filled with helium to a volume of
345 L at 33 °C and 759 mmHg The balloon
ascends to an altitude where the pressure is 494
mmHg and the temperature is -31 °C
Part
What is the volume of the balloon at this altitude?
Express your answer using three significant figures.
V =
Submit
Request Answer
Provide Feedback
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning