(a) Would silver react with dilute sulfuric acid? explain why or why not. (b) Would magnesium react with dilute sulfuric acid? explain why or why not.
(a) Would silver react with dilute sulfuric acid? explain why or why not. (b) Would magnesium react with dilute sulfuric acid? explain why or why not.
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter5: Chemical Reactions
Section: Chapter Questions
Problem 5.18E
Related questions
Question
1. Additial Exercies
(a) Would silver react with dilute sulfuric acid? explain why or why not.
(b) Would magnesium react with dilute sulfuric acid? explain why or why not.
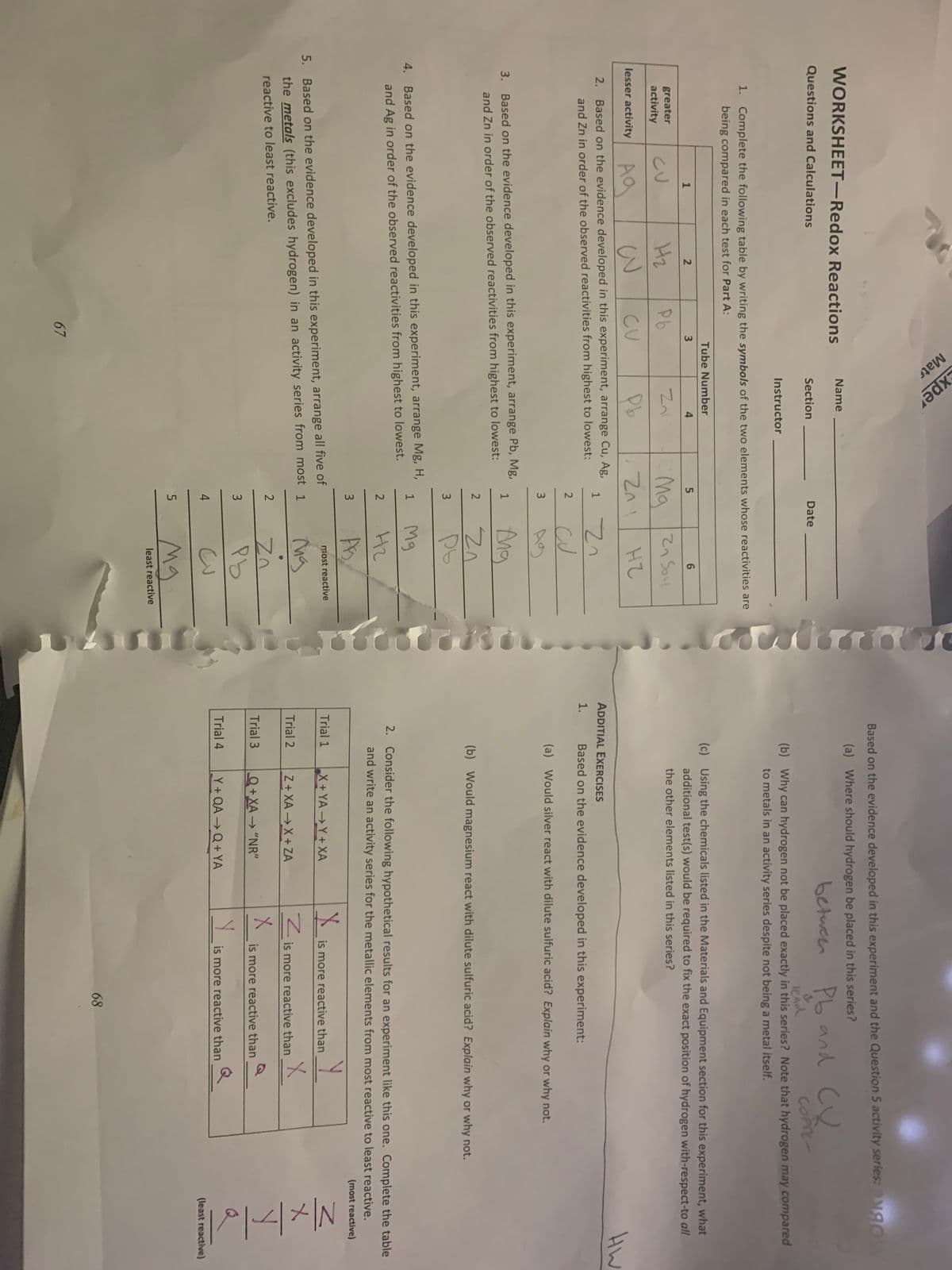
Transcribed Image Text:Mats
ədx
4»
Based on the evidence developed in this experiment and the Question 5 activity series:M gO
WORKSHEET-Redox Reactions
(a) Where should hydrogen be placed in this series?
Name
betueen Pb and Cu
co-
Questions and Calculations
Section
Date
lead
(b) Why can hydrogen not be placed exactly in this series? Note that hydrogen may compared
to metals in an activity series despite not being a metal itself.
Instructor
1. Complete the following table by writing the symbols of the two elements whose reactivities are
being compared in each test for Part A:
Tube Number
(c) Using the chemicals listed in the Materials and Equipment section for this experiment, what
additional test(s) would be required to fix the exact position of hydrogen with-respect-to all
4
6.
greater
CU
Hz
Pb
Ma 2n Soul
the other elements listed in this series?
activity
3
CU
Pb
lesser activity
HW
2. Based on the evidence developed in this experiment, arrange Cu, Ag,
1
1 Z7
ADDITIAL EXERCISES
and Zn in order of the observed reactivities from highest to lowest:
1.
Based on the evidence developed in this experiment:
2
(a) Would silver react with dilute sulfuric acid? Explain why or why not.
3
3. Based on the evidence developed in this experiment, arrange Pb, Mg,
1
Ang
and Zn in order of the observed reactivities from highest to lowest:
(b) Would magnesium react with dilute sulfuric acid? Explain why or why not.
3.
4. Based on the evidence developed in this experiment, arrange Mg, H,
Mg
1
and Ag in order of the observed reactivities from highest to lowest.
2. Consider the following hypothetical results for an experiment like this one. Complete the table
H2
and write an activity series for the metallic elements from most reactive to least reactive.
3
(most reactive)
most reactive
Trial 1
X+YAY+XA
is more reactive than
5. Based on the evidence developed in this experiment, arrange all five of
the metals (this excludes hydrogen) in an activity series from most 1
Trial 2
Z+ XA X+ZA
Z is more reactive than
reactive to least reactive.
Trial 3
Q+XA→ "NR"
is more reactive than
3
Trial 4
Y + QA → Q + YA
Y is more reactive than
(least reactive)
Mg
least reactive
68
67
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning