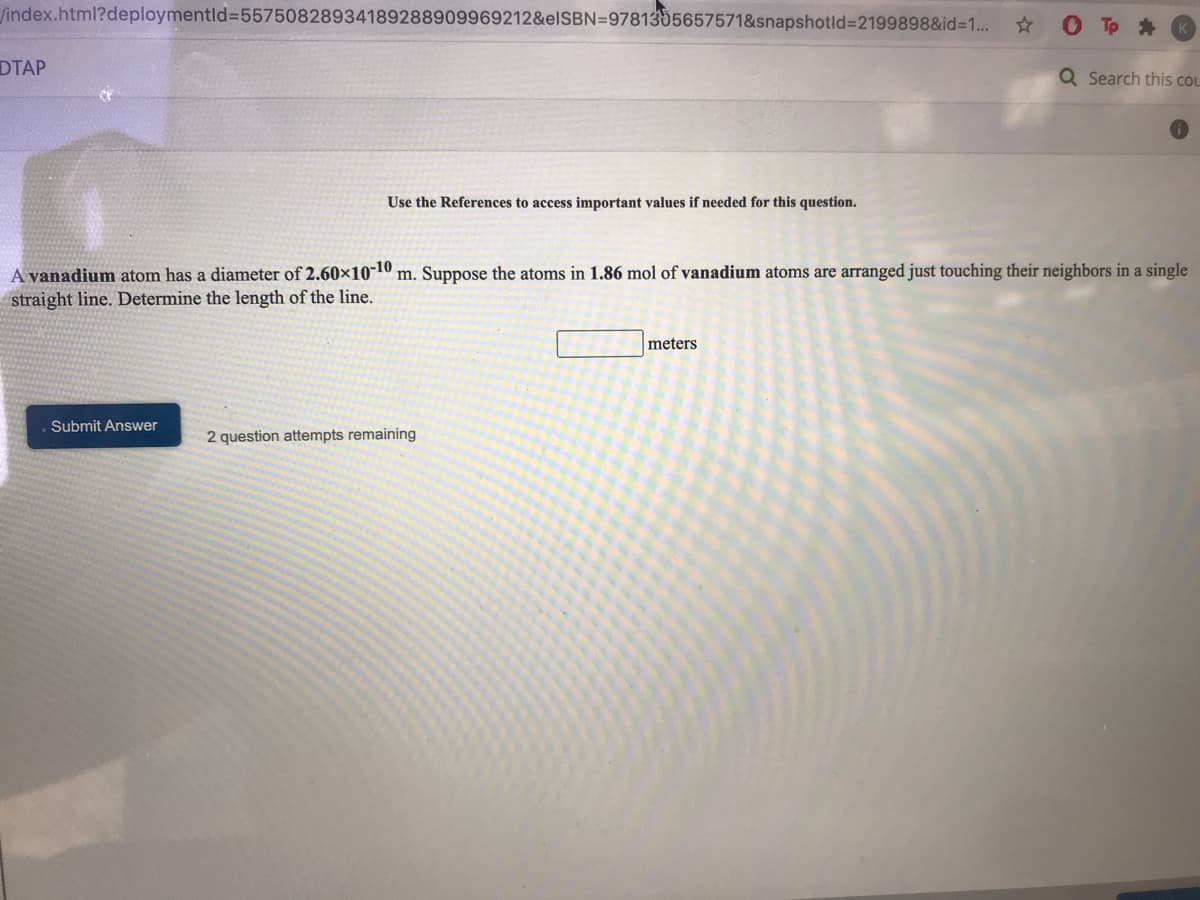
A yanadium atom has a diameter of 2.60×10-10 m. Suppose the atoms in 1.86 mol of vanadium atoms are arranged just touching their neighbors in a single straight line. Determine the length of the line. meters
A yanadium atom has a diameter of 2.60×10-10 m. Suppose the atoms in 1.86 mol of vanadium atoms are arranged just touching their neighbors in a single straight line. Determine the length of the line. meters
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 104AE
Related questions
Question
Transcribed Image Text:/index.html?deploymentld%=55750828934189288909969212&elSBN=978135657571&snapshotld%32199898&id%3D1.
O TP *
DTAP
Q Search this cou
Use the References to access important values if needed for this question.
A vanadium atom has a diameter of 2.60×10-10 m. Suppose the atoms in 1.86 mol of vanadium atoms are arranged just touching their neighbors in a single
straight line. Determine the length of the line.
meters
.Submit Answer
2 question attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax