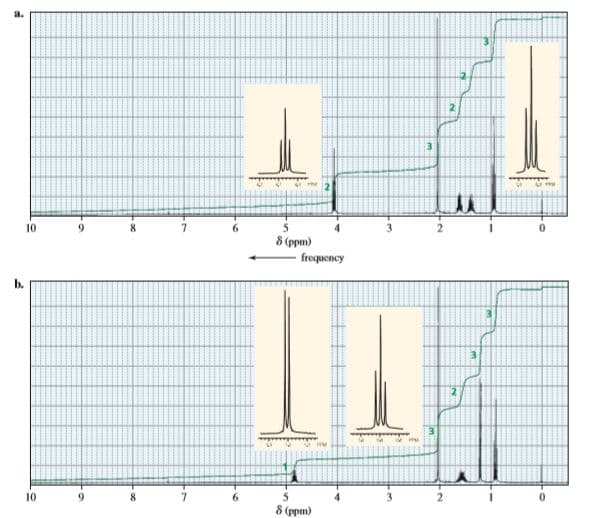
The following 1H NMR spectra are for four compounds, each with molecular formula of C6H12O2. Identify the compounds
Step 1
- The DBU of a compound with molecular formula C6H12O2is as follows:
DBU = 6 – 12/2 + 1 = 1 i.e. unsaturation present. This suggests that the carbonyl group is present.
The 12 hydrogens are divided into 5 sets of Hydrogens.
The given 1H NMR spectrum has:
3H with triplet splitting at 0.9 ppm (at base value)
2H’s with multiplet splitting at 1.4 and 1.6 ppm (the slight increment arises due to increment of the presence of group).
3H with singlet has a peak at 2.0 ppm this suggests that no hydrogen is present adjacent and increment is raised due to the presence of carbonyl group.
Lastly, 2H’s have triplet splitting at 4.1 ppm, such a huge increment arises due to direct attachment of Electronegative group (O-atom).
Thus, the structure of the compound would be:
Step by step
Solved in 2 steps with 2 images






