a. calcium oxide b. potassium chloride c. mercury(I) oxide d. zinc fluoride e. barium hydroxide f. sodium peroxide g. chromium(III) bromide h. gold(III) cyanide i. strontium phosphide j. cobalt(II) sulfide k. aluminum oxide 1. chromium(VI) oxide m. copper(II) oxide n. lead(IV) sulfide o. copper(II) cyanide p. silver iodide q. nickel(II) sulfide r. mercury(II) nitride s. lithium nitride t. calcium carbide
a. calcium oxide b. potassium chloride c. mercury(I) oxide d. zinc fluoride e. barium hydroxide f. sodium peroxide g. chromium(III) bromide h. gold(III) cyanide i. strontium phosphide j. cobalt(II) sulfide k. aluminum oxide 1. chromium(VI) oxide m. copper(II) oxide n. lead(IV) sulfide o. copper(II) cyanide p. silver iodide q. nickel(II) sulfide r. mercury(II) nitride s. lithium nitride t. calcium carbide
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter21: The Chemistry Of The Main Group Elements
Section: Chapter Questions
Problem 119IL
Related questions
Question
1. Complete the following problems from the “Inorganic Nomenclature”
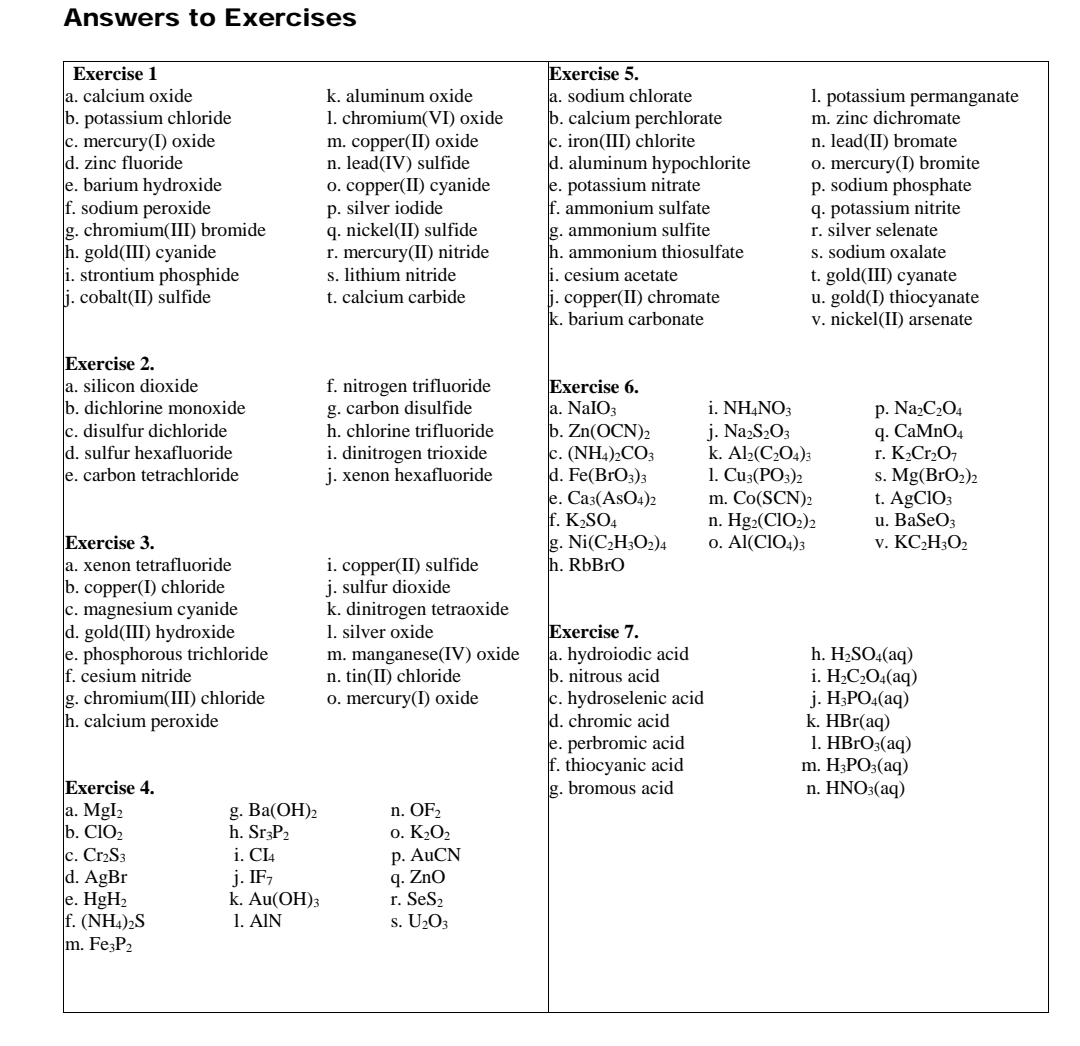
Transcribed Image Text:Answers to Exercises
Exercise 5.
a. sodium chlorate
b. calcium perchlorate
c. iron(III) chlorite
d. aluminum hypochlorite
e. potassium nitrate
f. ammonium sulfate
g. ammonium sulfite
h. ammonium thiosulfate
i. cesium acetate
Exercise 1
a. calcium oxide
b. potassium chloride
c. mercury(I) oxide
d. zinc fluoride
e. barium hydroxide
f. sodium peroxide
g. chromium(III) bromide
h. gold(III) cyanide
i. strontium phosphide
lj. cobalt(II) sulfide
1. potassium permanganate
m. zinc dichromate
k. aluminum oxide
1. chromium(VI) oxide
m. copper(II) oxide
n. lead(IV) sulfide
0. сopper(II) суаnide
p. silver iodide
q. nickel(II) sulfide
r. mercury(II) nitride
s. lithium nitride
n. lead(II) bromate
0. mercury(I) bromite
p. sodium phosphate
q. potassium nitrite
r. silver selenate
s. sodium oxalate
t. gold(III) cyanate
u. gold(I) thiocyanate
v. nickel(II) arsenate
j. copper(II) chromate
k. barium carbonate
t. calcium carbide
Exercise 2.
a. silicon dioxide
b. dichlorine monoxide
c. disulfur dichloride
d. sulfur hexafluoride
e. carbon tetrachloride
f. nitrogen trifluoride
g. carbon disulfide
Exercise 6.
a. NaIO3
b. Zn(OCN)2
c. (NH4)2CO3
d. Fe(BrO3)3
e. Саз (ASO4)2
f. K,SO4
g. Ni(C,H3O2)4
h. RbBrO
i. NΗΝO
j. NazS2O3
k. Al2(C2O4)3
1. Cu:(PОз)2
m. Co(SCN)2
n. Hg2(CIO2)2
o. Al(CIO4)3
p. NazC2O4
9. СаMnO4
r. K2CrO7
s. Mg(BrO2)2
t. AgClO3
u. BaSeO3
v. KC,H3O2
h. chlorine trifluoride
i. dinitrogen trioxide
j. xenon hexafluoride
Exercise 3.
a. xenon tetrafluoride
b. copper(I) chloride
c. magnesium cyanide
d. gold(III) hydroxide
e. phosphorous trichloride
f. cesium nitride
g. chromium(III) chloride
h. calcium peroxide
i. copper(II) sulfide
j. sulfur dioxide
k. dinitrogen tetraoxide
1. silver oxide
m. manganese(IV) oxide
n. tin(II) chloride
0. mercury(I) oxide
Exercise 7.
a. hydroiodic acid
b. nitrous acid
c. hydroselenic acid
d. chromic acid
e. perbromic acid
f. thiocyanic acid
g. bromous acid
h. H2SO4(aq)
i. H¿C¿O4(aq)
j. H3PO4(aq)
k. HBr(aq)
1. HBrO3(aq)
m. H3PO3(aq)
n. HNO3(aq)
Exercise 4.
a. MgI2
b. CIO2
c. Cr2S3
d. AgBr
e. HgH2
f. (NH4)2S
m. Fe;P2
g. Ba(ОН)2
h. Sr3P2
i. Cl4
j. IF,
k. Au(OH)3
1. ΑIΝ
n. OF2
o. K2O2
p. AuCN
q. ZnO
r. SeS2
s. U2O3
Expert Solution
Step 1
Note: As per our guidelines, we are supposed to answer only the first question (i.e. exercise 1). Kindly repost other questions separately.
For the naming of inorganic compounds, first, write the name of the cation and then write the name of the anion.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning