a. Calculate the Natural Abundance of 4°X and enter it into the chart. b. Calculate the isotopic mass of 40X and enter it into the chart. Mass (amu) Natural Abundance Symbol (%) 0.337 35.96755 36X 38X 40X Average 37.96272 0.063 39.948
a. Calculate the Natural Abundance of 4°X and enter it into the chart. b. Calculate the isotopic mass of 40X and enter it into the chart. Mass (amu) Natural Abundance Symbol (%) 0.337 35.96755 36X 38X 40X Average 37.96272 0.063 39.948
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 25Q: The atomic masses in die periodic table are relative masses and average masses. Explain.
Related questions
Question
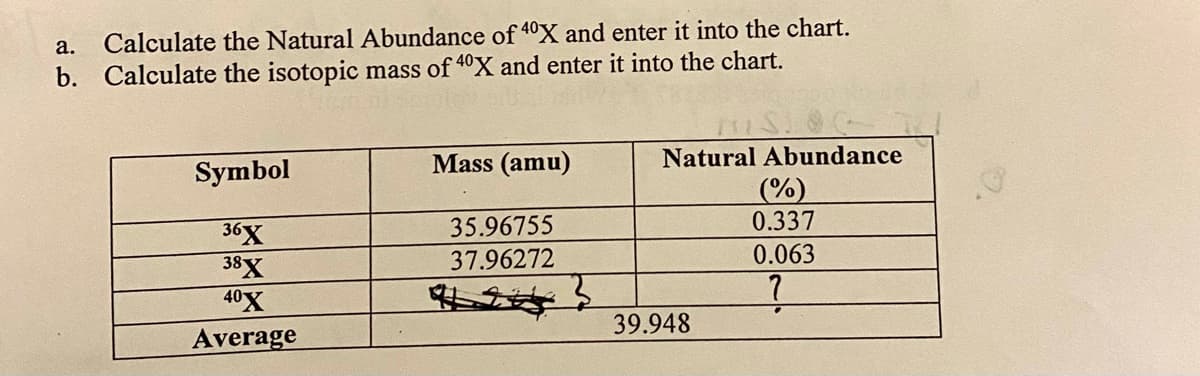
Transcribed Image Text:а.
Calculate the Natural Abundance of 40X and enter it into the chart.
b.
Calculate the isotopic mass of 40X and enter it into the chart.
Mass (amu)
Natural Abundance
Symbol
(%)
0.337
35.96755
36X
38X
40X
Average
37.96272
0.063
39.948
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning