The weight percent of silicon in six different rock samples, each containing different amounts of silicon, was measured by two different methods. The results are given in the table. Sample Method A Si wt% | Method B Si wt% 1 11.570 11.490 15.380 15.310 3 17.950 17.810 4 22.840 22.810 5 25.110 25.200 6 27.070 27.050 Determine tcalc• t calc = 3.122
The weight percent of silicon in six different rock samples, each containing different amounts of silicon, was measured by two different methods. The results are given in the table. Sample Method A Si wt% | Method B Si wt% 1 11.570 11.490 15.380 15.310 3 17.950 17.810 4 22.840 22.810 5 25.110 25.200 6 27.070 27.050 Determine tcalc• t calc = 3.122
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter4: Stoichiometry: Quantitative Information About Chemical Reactions
Section: Chapter Questions
Problem 102GQ: Cloth can be waterproofed by coating it with a silicone layer. This is done by exposing the cloth to...
Related questions
Question
I am struggling with finding tcalc, I know it is not 1.64
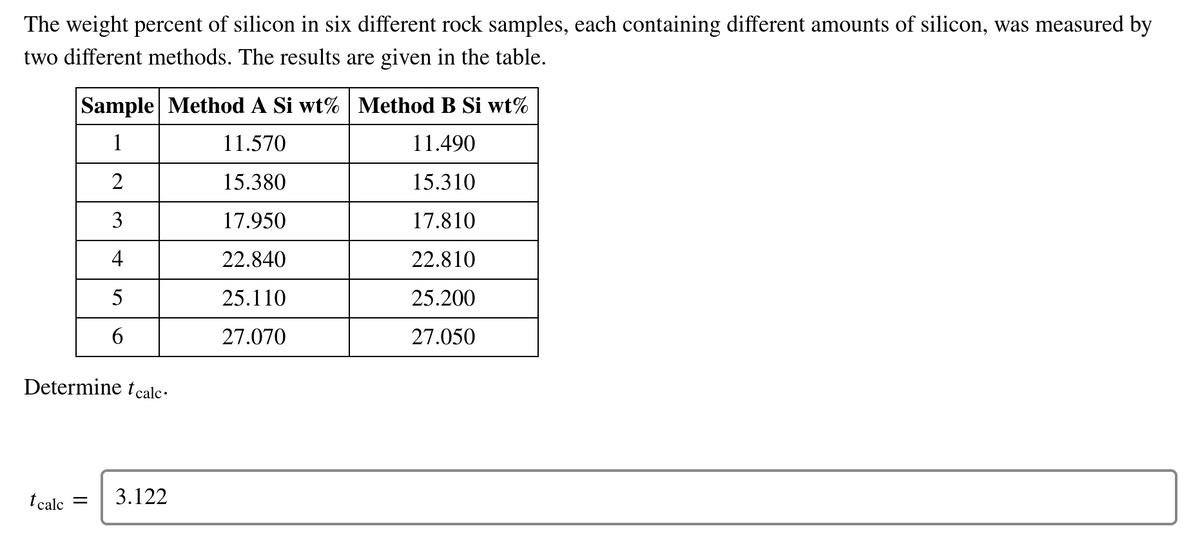
Transcribed Image Text:The weight percent of silicon in six different rock samples, each containing different amounts of silicon, was measured by
two different methods. The results are given in the table.
Sample Method A Si wt% Method B Si wt%
1
11.570
11.490
2
15.380
15.310
3
17.950
17.810
4
22.840
22.810
5
25.110
25.200
27.070
27.050
Determine tecalc·
t calc =
3.122
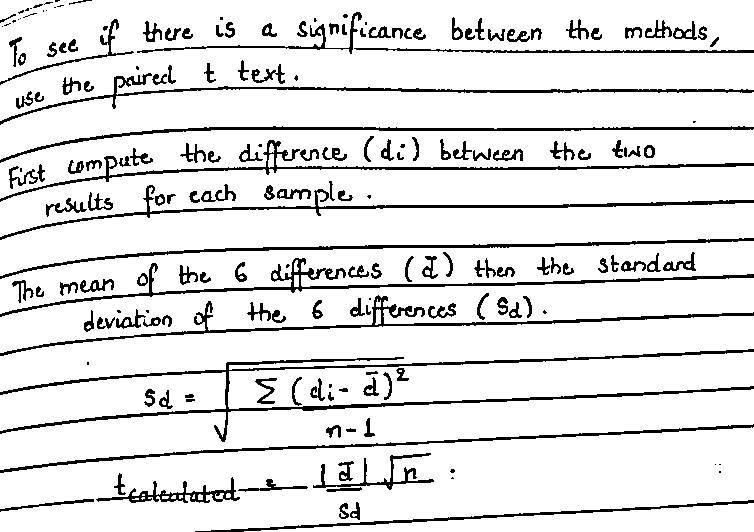
Expert Solution
Step 1
Solution
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
