A. Chemical В. C. Formula D. Balance E. F. IUPAC name of the compound General Type ( Binary, Ternary or Diatomic) Specific Composition of the common name of the reaction of the of the Compound compound Compound copound Nitrogen trihydride Aluminum chloride KBr NH.+ Mn0,
A. Chemical В. C. Formula D. Balance E. F. IUPAC name of the compound General Type ( Binary, Ternary or Diatomic) Specific Composition of the common name of the reaction of the of the Compound compound Compound copound Nitrogen trihydride Aluminum chloride KBr NH.+ Mn0,
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 52QAP: Write a balanced equation for the reaction between (a) dihydrogen sulfide and sulfur dioxide gases...
Related questions
Question
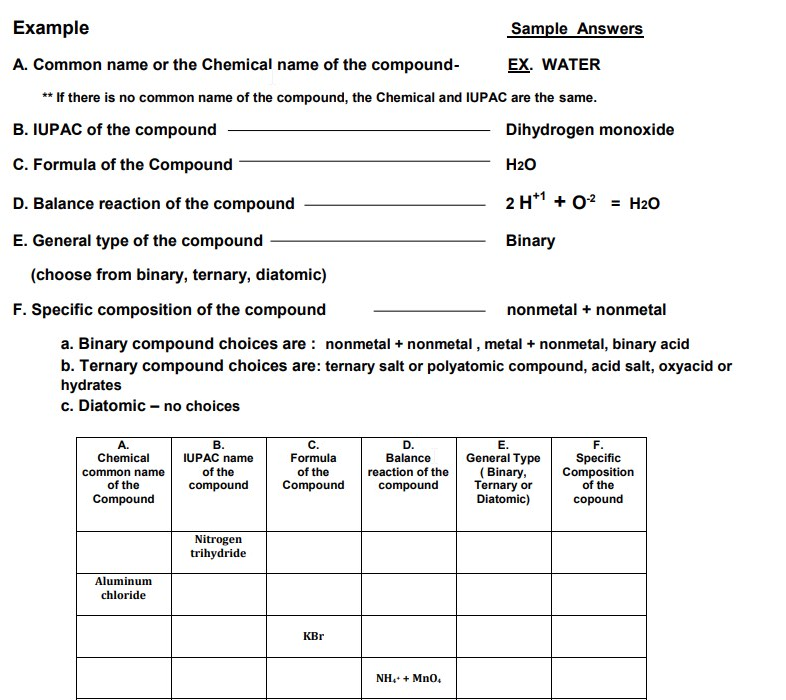
Transcribed Image Text:Example
Sample Answers
A. Common name or the Chemical name of the compound-
EX. WATER
** If there is no common name of the compound, the Chemical and IUPAC are the same.
B. IUPAC of the compound
Dihydrogen monoxide
C. Formula of the Compound
H2O
D. Balance reaction of the compound
2 H*1 + 0? = H2O
E. General type of the compound
Binary
(choose from binary, ternary, diatomic)
F. Specific composition of the compound
nonmetal + nonmetal
a. Binary compound choices are : nonmetal + nonmetal , metal + nonmetal, binary acid
b. Ternary compound choices are: ternary salt or polyatomic compound, acid salt, oxyacid or
hydrates
c. Diatomic – no choices
A.
Chemical
В.
IUPAC name
C.
Formula
D.
Balance
Е.
General Type
( Binary,
Ternary or
Diatomic)
F.
Specific
Composition
of the
common name
of the
of the
reaction of the
of the
compound
Compound
compound
Compound
copound
Nitrogen
trihydride
Aluminum
chloride
KBr
NH,+ + Mn0,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax