Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
ChapterA1: Evaluation Of Analytical Data
Section: Chapter Questions
Problem A1.22QAP
Related questions
Question
100%
Solution Q number 1
Transcribed Image Text:1 %7. |1.
Assignment 1_Chem...
->
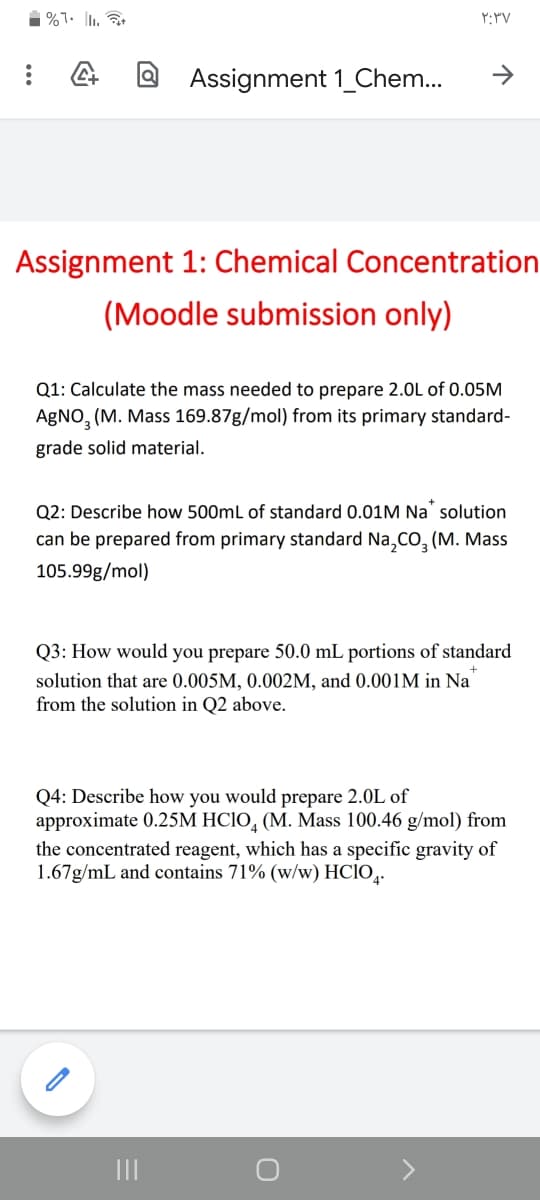
Assignment 1: Chemical Concentration
(Moodle submission only)
Q1: Calculate the mass needed to prepare 2.0L of 0.05M
AgNO, (M. Mass 169.87g/mol) from its primary standard-
grade solid material.
Q2: Describe how 500mL of standard 0.01M Na solution
can be prepared from primary standard Na,CO, (M. Mass
105.99g/mol)
Q3: How would you prepare 50.0 mL portions of standard
solution that are 0.005M, 0.002M, and 0.001M in Na
from the solution in Q2 above.
Q4: Describe how you would prepare 2.0L of
approximate 0.25M HC1O, (M. Mass 100.46 g/mol) from
the concentrated reagent, which has a specific gravity of
1.67g/mL and contains 71% (w/w) HCIO,.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT