a. Cyclooctatetraene (COT) is shown below. use the Hückel molecular orbital theory "polygon-in-circle," method to draw a diagram of the relative energies of the n molecular orbitals for COT (you should also fill out this diagram with the correct number of t electrons and indicate which molecular orbitals are bonding, nonbonding, and antibonding). Based solely on the MO diagram you may have learned in class), would this hypothetical planar COT be aromatic, anti-aromatic, or non-aromatic? Assume the molecule is planar, (do not consider anything else about the structure
a. Cyclooctatetraene (COT) is shown below. use the Hückel molecular orbital theory "polygon-in-circle," method to draw a diagram of the relative energies of the n molecular orbitals for COT (you should also fill out this diagram with the correct number of t electrons and indicate which molecular orbitals are bonding, nonbonding, and antibonding). Based solely on the MO diagram you may have learned in class), would this hypothetical planar COT be aromatic, anti-aromatic, or non-aromatic? Assume the molecule is planar, (do not consider anything else about the structure
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter10: Molecular Structure And Bonding Theories
Section: Chapter Questions
Problem 10.101QE: The molecular orbital diagram of NO shown in Figure 10.47 also applies to OF. Draw the complete...
Related questions
Question
Parts A,B, and C are all apart of this problem.
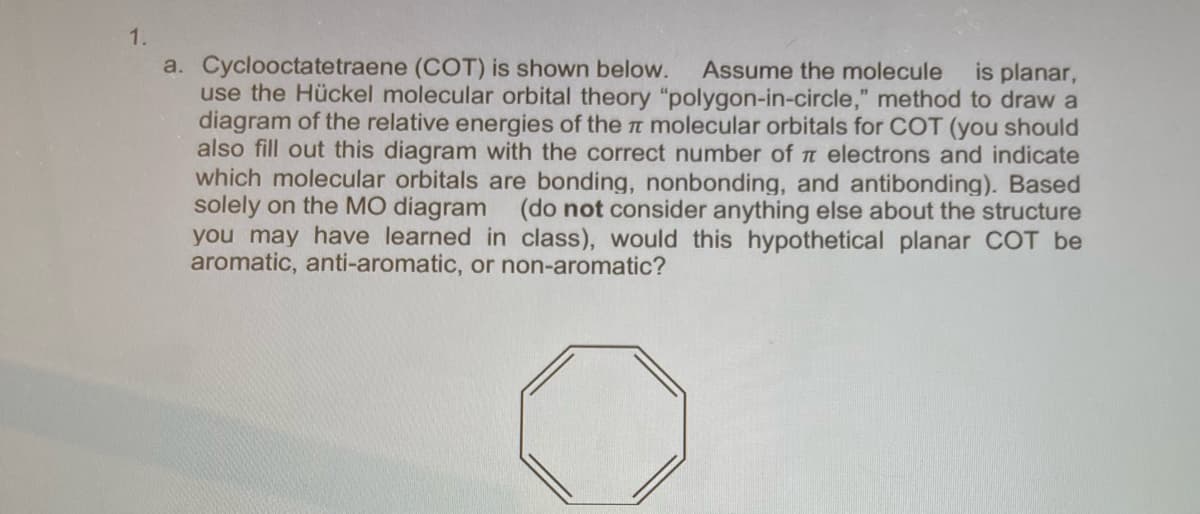
Transcribed Image Text:1.
a. Cyclooctatetraene (COT) is shown below.
use the Hückel molecular orbital theory "polygon-in-circle," method to draw a
diagram of the relative energies of the t molecular orbitals for COT (you should
also fill out this diagram with the correct number of t electrons and indicate
which molecular orbitals are bonding, nonbonding, and antibonding). Based
solely on the MO diagram (do not consider anything else about the structure
you may have learned in class), would this hypothetical planar COT be
aromatic, anti-aromatic, or non-aromatic?
Assume the molecule
is planar,
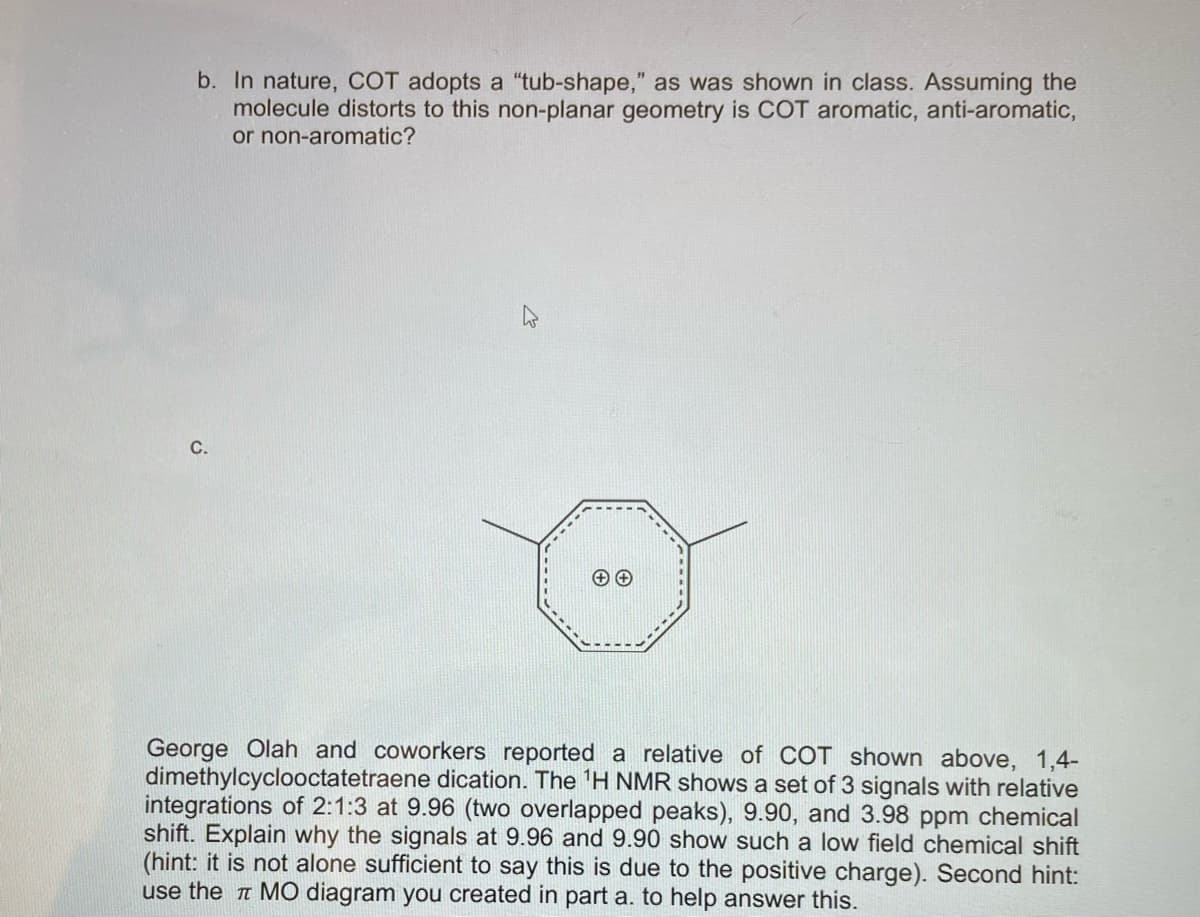
Transcribed Image Text:b. In nature, COT adopts a "tub-shape," as was shown in class. Assuming the
molecule distorts to this non-planar geometry is COT aromatic, anti-aromatic,
or non-aromatic?
C.
George Olah and coworkers reported a relative of COT shown above, 1,4-
dimethylcyclooctatetraene dication. The 'H NMR shows a set of 3 signals with relative
integrations of 2:1:3 at 9.96 (two overlapped peaks), 9.90, and 3.98 ppm chemical
shift. Explain why the signals at 9.96 and 9.90 show such a low field chemical shift
(hint: it is not alone sufficient to say this is due to the positive charge). Second hint:
use the a MO diagram you created in part a. to help answer this.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning