A. Determine the Fhkl Factor of the ionic crystal NaCl, assuming the scattering factors for Na and Cl atoms are fNa and fcl B. calculate Fhkl for field (111), (200)
A. Determine the Fhkl Factor of the ionic crystal NaCl, assuming the scattering factors for Na and Cl atoms are fNa and fcl B. calculate Fhkl for field (111), (200)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section: Chapter Questions
Problem 74QRT
Related questions
Question
I need the answer as soon as possible
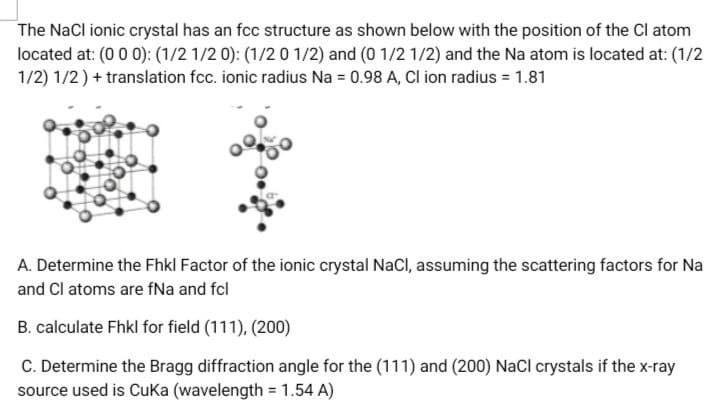
Transcribed Image Text:The NaCl ionic crystal has an fcc structure as shown below with the position of the Cl atom
located at: (0 0 0): (1/2 1/2 0): (1/2 0 1/2) and (0 1/2 1/2) and the Na atom is located at: (1/2
1/2) 1/2) + translation fcc. ionic radius Na = 0.98 A, Cl ion radius = 1.81
A. Determine the Fhkl Factor of the ionic crystal NaCl, assuming the scattering factors for Na
and Cl atoms are fNa and fcl
B. calculate Fhkl for field (111), (200)
C. Determine the Bragg diffraction angle for the (111) and (200) NaCl crystals if the x-ray
source used is Cuka (wavelength = 1.54 A)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 6 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,