a. Epichlorohydrin reacts with dimethyl malonate to give an unusual product NaOMe Meo b. Ketals can be brominated under acidic conditions too! H*, Bra Br
a. Epichlorohydrin reacts with dimethyl malonate to give an unusual product NaOMe Meo b. Ketals can be brominated under acidic conditions too! H*, Bra Br
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter19: Eas: Electrophilic Aromatic Substitution
Section: Chapter Questions
Problem 7CTQ
Related questions
Question
3
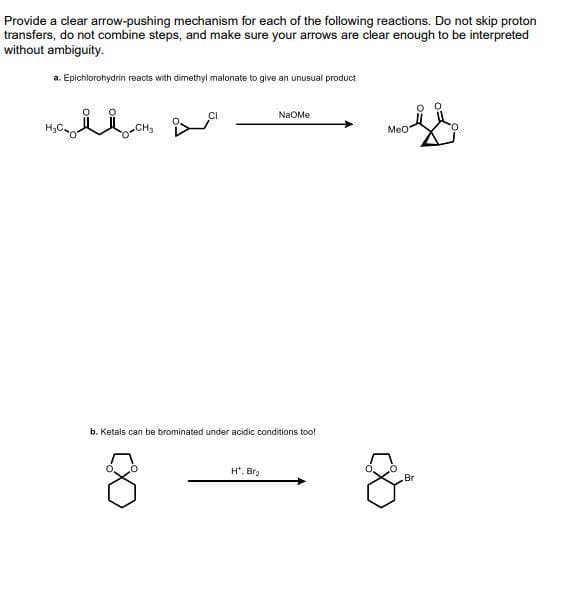
Transcribed Image Text:Provide a cdlear arrow-pushing mechanism for each of the following reactions. Do not skip proton
transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted
without ambiguity.
a. Epichlorohydrin reacts with dimethyl malonate to give an unusual product
NaOMe
MeO
b. Ketals can be brominated under acidic conditions too!
H*, Bra
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning