A. Extremophile microorganisms have been found that can thrive at extreme temperatures. Some bacteria have been documented to live in 80C water temperatures. Assuming the same ion concentrations from the table down below, calculate Nernst potential for K+. What would happen to this potential if you stored the bacteria in the fridge (4C)? B. Calculate the Nernst Potential for Ca+2 and Cl- using all the info from A
A. Extremophile microorganisms have been found that can thrive at extreme temperatures. Some bacteria have been documented to live in 80C water temperatures. Assuming the same ion concentrations from the table down below, calculate Nernst potential for K+. What would happen to this potential if you stored the bacteria in the fridge (4C)? B. Calculate the Nernst Potential for Ca+2 and Cl- using all the info from A
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter31: Immunochemistry
Section: Chapter Questions
Problem 31.82P
Related questions
Question
A.
Extremophile microorganisms have been found that can thrive at extreme
temperatures. Some bacteria have been documented to live in 80C water temperatures.
Assuming the same ion concentrations from the table down below, calculate Nernst potential for K+.
What would happen to this potential if you stored the bacteria in the fridge (4C)?
B. Calculate the Nernst Potential for Ca+2 and Cl- using all the info from A
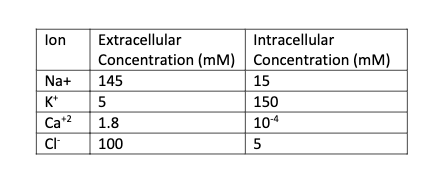
Transcribed Image Text:lon
Extracellular
Intracellular
Concentration (mM) Concentration (mM)
145
Na+
15
K*
5
150
Ca+2
1.8
104
100
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning