A. Solubility Fill-in the table below with + (soluble) or – (insoluble). Dilute N2OH Sample H2O Dilute Cold Hot CHCI3 Ether CCI4 HCI C;HŞOH C2H5OH Coconut oil Palmitic acid
A. Solubility Fill-in the table below with + (soluble) or – (insoluble). Dilute N2OH Sample H2O Dilute Cold Hot CHCI3 Ether CCI4 HCI C;HŞOH C2H5OH Coconut oil Palmitic acid
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter18: Lipids
Section: Chapter Questions
Problem 18.73E: Fats belong to the class of organic compounds represented by the general formula, RCOOR', where R...
Related questions
Question
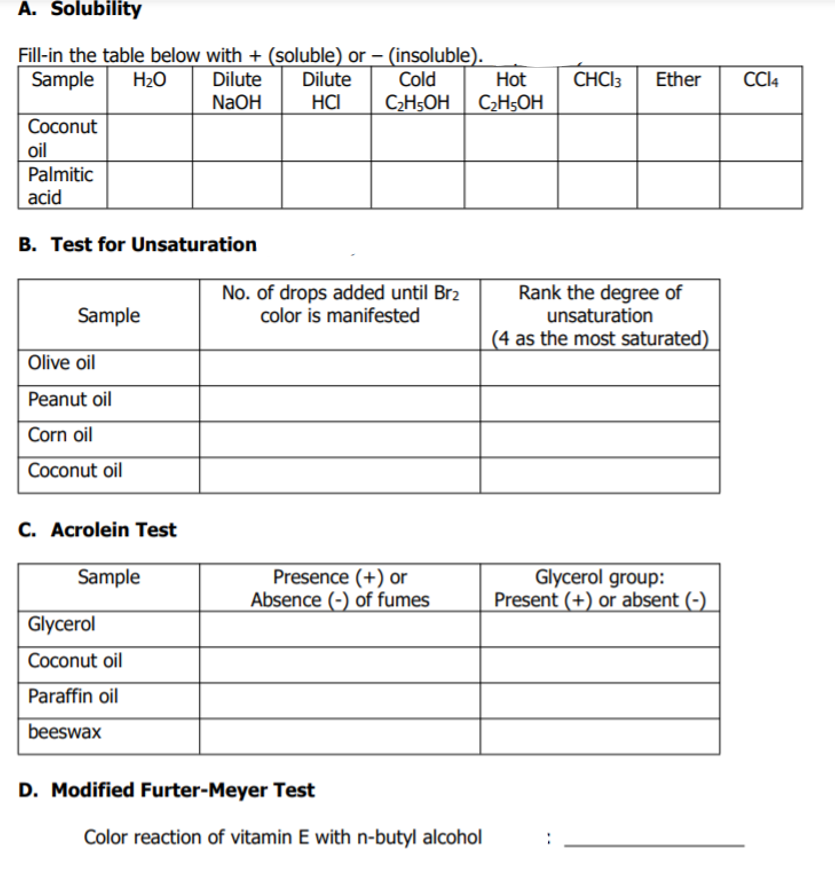
Transcribed Image Text:A. Solubility
Fill-in the table below with + (soluble) or – (insoluble).
Dilute
NaOH
Sample
H2O
Dilute
Cold
Hot
CHCI3
Ether
CI4
HCI
C;H5OH C2H5OH
Coconut
oil
Palmitic
acid
B. Test for Unsaturation
No. of drops added until Br2
color is manifested
Rank the degree of
unsaturation
Sample
(4 as the most saturated)
Olive oil
Peanut oil
Corn oil
Coconut oil
C. Acrolein Test
Presence (+) or
Absence (-) of fumes
Glycerol group:
Present (+) or absent (-)
Sample
Glycerol
Coconut oil
Paraffin oil
beeswax
D. Modified Furter-Meyer Test
Color reaction of vitamin E with n-butyl alcohol

Transcribed Image Text:QUESTIONS:
1. Based on your experiment, enumerate the best solvents for lipids.
a)
c)
b)
d)
2. Classify the following lipids as unsaturated or saturated.
a) Oleic acid
b) Linoleic acid
3. Give the formula of the pungent substance produced when vegetable oil is heated
until it becomes smoky.
4. Give the expected result (positive or negative) of the following lipids with acrolein
test.
a) Corn oil
b) Palmitic acid
5. Give one group of compounds which contributes to the keeping quality of fats.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div