a. The graph shows that the basic form has a maximum absorption at Amax, while the acidic has its minimum absorption at Amax. Explain why the basic form and acidic form have opposite absorptions at this specific wavelength. b. What is the importance of determining the Amax?
a. The graph shows that the basic form has a maximum absorption at Amax, while the acidic has its minimum absorption at Amax. Explain why the basic form and acidic form have opposite absorptions at this specific wavelength. b. What is the importance of determining the Amax?
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter14: Applications Of Ultraviolet-visible Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 14.9QAP
Related questions
Question
100%
Please do not use the answer I notice floating around the web. I am seeking clarity concerning this question.
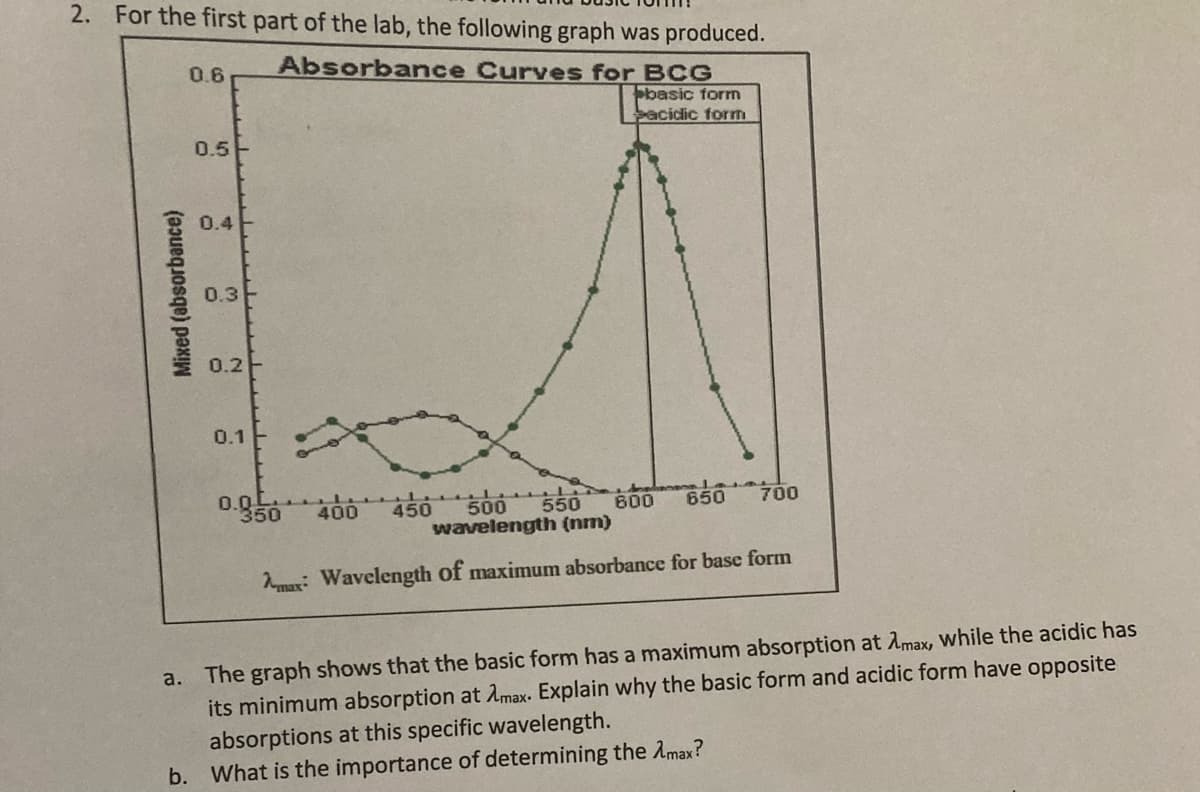
Transcribed Image Text:2. For the first part of the lab, the following graph was produced.
0.6
Absorbance Curves for BCG
basic forn
bacidic form
0.5
0.4
0.3
0.2-
0.1
0.0
350
4505
500
550
600
650
700
400
wavelength (nm)
max: Wavelength of maximum absorbance for base form
a. The graph shows that the basic form has a maximum absorption at Amax, while the acidic has
its minimum absorption at Amax. Explain why the basic form and acidic form have opposite
absorptions at this specific wavelength.
b. What is the importance of determining the Amax?
Mixed (absorbance)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
