a. Use strain energy increments in the OWL Table Reference (see References button, Strain Energy Increments) to calculate the energy difference between the two chair conformations of the compound below. b. Specify substituent positions (axial or equatorial) in the more stable chair. c. Estimate the percent of the more stable chair at equilibrium at 25°C. (To determine the percent of the more stable chair at equilibrium, first calculate Keg and then use this value to find the percentage.) CH3 H3C он Answers: a. The energy difference is kJ/mol.
a. Use strain energy increments in the OWL Table Reference (see References button, Strain Energy Increments) to calculate the energy difference between the two chair conformations of the compound below. b. Specify substituent positions (axial or equatorial) in the more stable chair. c. Estimate the percent of the more stable chair at equilibrium at 25°C. (To determine the percent of the more stable chair at equilibrium, first calculate Keg and then use this value to find the percentage.) CH3 H3C он Answers: a. The energy difference is kJ/mol.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter7: Cycloalkanes
Section: Chapter Questions
Problem 17E: Build a model of methylcyclohexane, and use the model to complete the following Newmanprojections of...
Related questions
Question
4
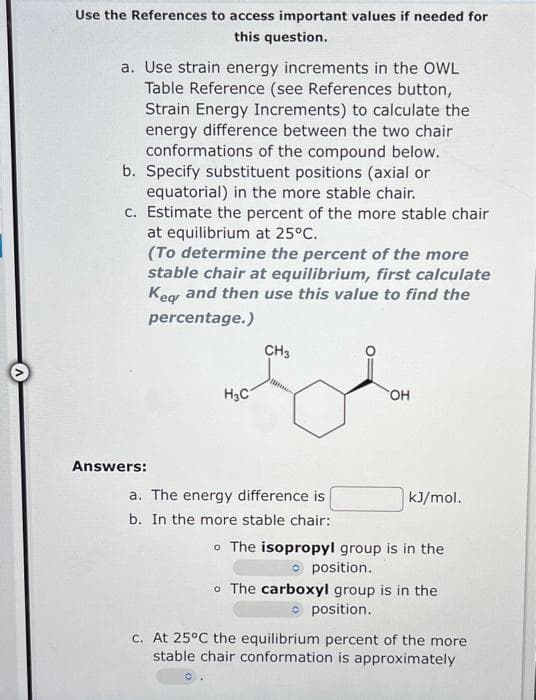
Transcribed Image Text:Use the References to access important values if needed for
this question.
a. Use strain energy increments in the OWL
Table Reference (see References button,
Strain Energy Increments) to calculate the
energy difference between the two chair
conformations of the compound below.
b. Specify substituent positions (axial or
equatorial) in the more stable chair.
c. Estimate the percent of the more stable chair
at equilibrium at 25°C.
(To determine the percent of the more
stable chair at equilibrium, first calculate
Keg and then use this value to find the
percentage.)
CH3
H3C
HO.
Answers:
a. The energy difference is
kJ/mol.
b. In the more stable chair:
o The isopropyl group is in the
O position.
o The carboxyl group is in the
O position.
c. At 25°C the equilibrium percent of the more
stable chair conformation is approximately
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning