A. Write a net ionic equation for the reaction that occurs when excess nitric acid (aq) and copper(II) carbonate are combined. + B. Write a net ionic equation for the reaction that occurs when calcium sulfide and excess hydrochloric acid (aq) are combined. + + C. Write a net ionic equation for the reaction that occurs when potassium sulfite (aq) and excess nitric acid (aq) are combined. Note: Sulfites follow the same solubility trends as sulfates.
A. Write a net ionic equation for the reaction that occurs when excess nitric acid (aq) and copper(II) carbonate are combined. + B. Write a net ionic equation for the reaction that occurs when calcium sulfide and excess hydrochloric acid (aq) are combined. + + C. Write a net ionic equation for the reaction that occurs when potassium sulfite (aq) and excess nitric acid (aq) are combined. Note: Sulfites follow the same solubility trends as sulfates.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 25QAP: Consider the following generic equation: H+(aq)+ B(aq)HB(aq)For which of the following pairs would...
Related questions
Question
I need help understanding how to do this homework question. I've been out sick so I don't even know how to even begin to attempt this question.
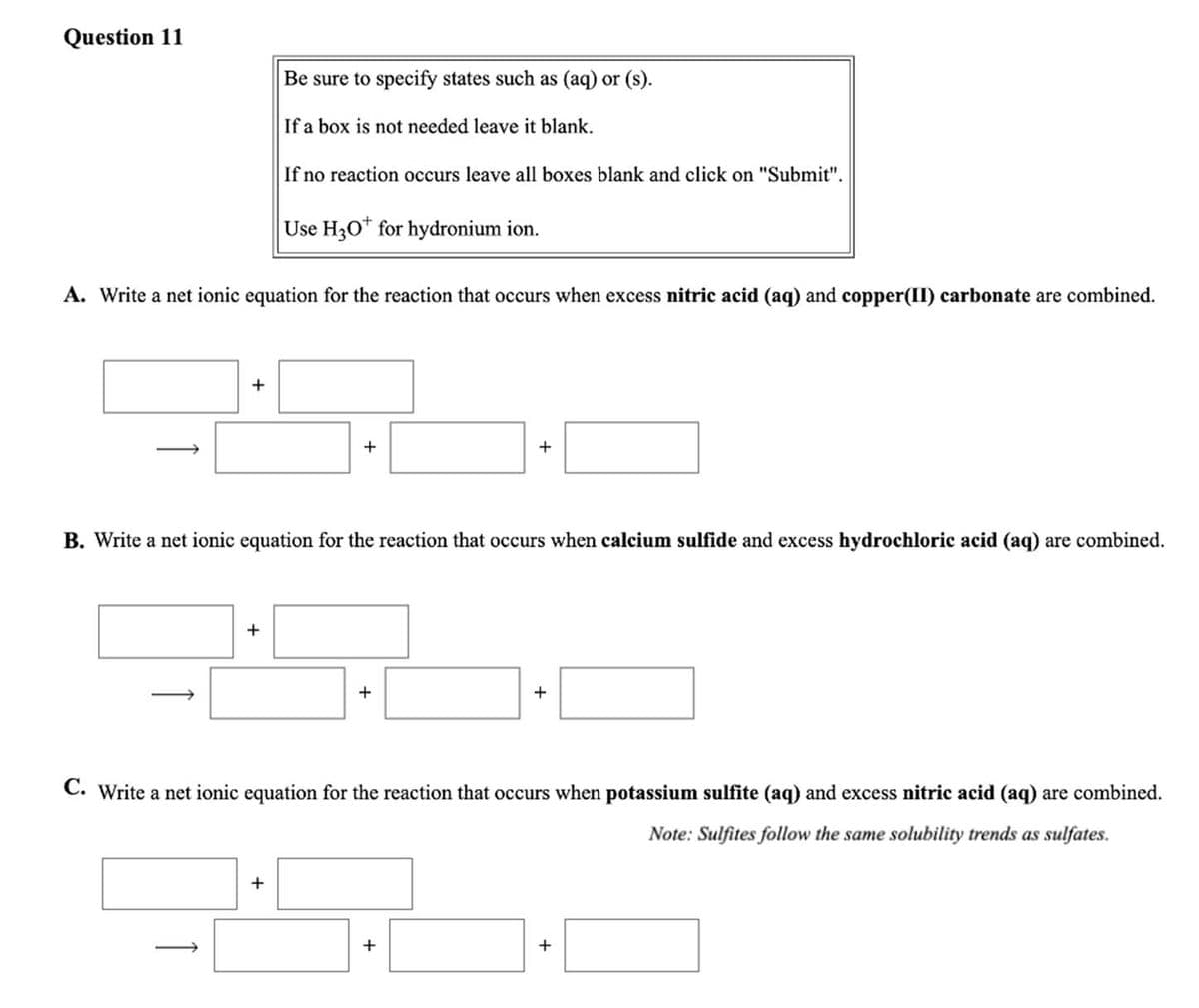
Transcribed Image Text:Question 11
Be sure to specify states such as (aq) or (s).
If a box is not needed leave it blank.
If no reaction occurs leave all boxes blank and click on "Submit".
Use H30* for hydronium ion.
A. Write a net ionic equation for the reaction that occurs when excess nitric acid (aq) and copper(II) carbonate are combined.
+
+
B. Write a net ionic equation for the reaction that occurs when calcium sulfide and excess hydrochloric acid (aq) are combined.
+
+
+
C. Write a net ionic equation for the reaction that occurs when potassium sulfite (aq) and excess nitric acid (aq) are combined.
Note: Sulfites follow the same solubility trends as sulfates.
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning